
High Frequency Oscillator Ventilation (HFOV) a
new strategy in the treatment
of patients with the Acute Respiratory Distress
Syndrome and low lung
compliance pathologies
Amanda Alves*
Interna Internato Complementar Medicina Interna
S. Medicina, Hospital Garcia de Orta
Almada,
Portugal
Abstract
Several lung protective ventilator strategies for treating patients with
ARDS have been investigated. These strategies aim to reverse atelectasis by
increasing alveolar recruitment, without overdistension of the existing healthy
alveolar units. Both derecruitment and overdistension appear to play an active
role in ongoing acute lung injury (ALI). Such ventilator strategies, using
conventional modes of ventilation, have been limited by the risk of barotrauma,
haemodynamic compromise and severe carbon dioxide (CO2) retention.
HFOV is a ventilatory mode in which high
frequency, low amplitude, pressure oscillations of gas at 5-15 Hz (up to 900
breaths/min), are generated in the airways, resulting in high mean airway
pressures and low tidal volumes (Vt) of 1-2ml/Kg. Both the
inspiratory and expiratory phases of ventilation are active. A high bias flow
of fresh gas is applied (20-60L/min).Gas exchange is achieved utilising
sub-deadspace tidal volumes, and as such may provide a less traumatic way of
recruiting and stabilising lung volumes. Five mechanisms of gas transport are
thought to be important when using HFOV. These are bulk axial flow,
interregional gas mixing, axial and radial augmented dispersion (Taylor
dispersion), convective dispersion and molecular diffusion. Efficient gas
transport seems to involve all five mechanisms and the overall coefficient of
gas transport during HFOV is a combined function of Vt2 and frequency. HFOV is coupled to a lung volume
optimisation protocol, which initially uses high mean airway pressures (‰AW),
to achieve optimal lung volumes via active alveolar recruitment. Optimising
lung volume, improves gas exchange and reduces shear stress forces between
expanded and collapsed lung units.
HFOV was originally used as a rescue technique in neonates with ARDS
when conventional ventilation failed. A number of studies indicate that early
application of this ventilatory mode may significantly improve the outcome of
this serious lung condition and reduce the development of chronic complications
in ARDS survivors.
Key words: High Frequency Oscillator Ventilator, Acute Respiratory Distress
Syndrome
High Frequency Oscillator
Ventilation (HFOV)
Introduction
Patients suffering from the Acute Respiratory Distress Syndrome (ARDS)
pose a challenge to all intensivists. The multifactorial origins which lead to
the development of the “Leaky Lung” syndrome, together with the associated high
mortality of this condition, has motivated critical care doctors to develop a
number of lung protective ventilator strategies. The objectives of such
measures are to reverse atelectasis by increasing alveolar recruitment, without
overdistension of the existing healthy lung units. Examples of such strategies
are “optimal” positive end expiratory pressure (PEEP), inverse ratio
pressure-controlled ventilation, low tidal volumes (Vt) < 6ml/kg,
patient positional changes and permissive hypercapneia. These measures are
limited by the risk of barotrauma, haemodynamic compromise and severe carbon
dioxide (CO2) retention.
HFOV is a ventilatory mode in which high frequency, low amplitude,
pressure oscillations of gas, at 5-15 Hz (up to 900 breaths/min), are generated
in the airways, together with high mean airway pressures (‰AW) and low tidal volumes (1-2ml/Kg). Both the inspiratory and expiratory
phases of ventilation are active. CO2 elimination is separated from
oxygen (O2) delivery. CO2 removal is effected by using a
continuous high bias flow of fresh gas (20-60L/min). This ventilatory mode,
together with a lung volume optimisation protocol to expand atelectatic lung
regions, is a new strategy for the treatment of ARDS and low lung compliance
pathologies.
Gas Transport
Classical respiratory physiology considers gas transport to occur via
the concept of bulk axial flow. Two lung compartments are considered:
1. The lung compartment in which homogeneous alveolar gas is exchanged with
the blood by molecular diffusion
2. The dead space (VD )where no gas exchange occurs
Using this model, under stable conditions, gas exchange is proportional to alveolar ventilation (VA) and when VT£ VD no gas exchange occurs:
VA = VT - VD
( VT =
Tidal volume and VD =
Dead space)
Alternative theories for gas exchange have been proposed, during recent years,
in an attempt to understand how patients ventilate adequately, using HFOV
ventilation. Five different mechanisms have been proposed [1]:
1.
Transit Time
Profile
2.
Interregional Gas
Mixing
3.
Augmented
Dispersion (Taylor Dispersion)
4.
Asymmetric
Velocity Profiles
5.
Molecular
Diffusion
Transit Time Profile
Using the classic gas transport model, it is assumed that all gas moves
along the airways at an equal velocity, forming a uniform front across which no
gas exchange occurs. The lung compartment is not a uniform structure and
transit times to alveolar units will vary in proportion to the length of the
bronchial airways. Proximal alveolar
units with short transit times will therefore ventilate before distal units and
adequate gas exchange may occur in proximal units, by direct bulk convection,
when tidal volumes are low.
Interregional Gas Mixing
The time that alveolar units take to fill during ventilation depends on their
compliance (C) and resistance (R) to airflow:
T = R x C
(T = Time constant of a given lung unit, R = Resistance to airflow, C = Compliance of alveolar unit)
Consequently, units with short time constants (low compliance, low
resistance) fill and empty more rapidly. The concept of inhomogeneity of lung
regional time constants was first introduced by Otis et al. [2] He proposed
that asynchronous filling and emptying of alveolar units occurs and that at the
end of expiration, units with short time constants (fast units) are empty and
ready to fill, whereas units with long time constants (slow units) are still
emptying. Consequently, gas moves from slow units to fast units. During
inspiration the opposite occurs and gas moves from fast to slow units. This
movement has been named Pendelluft
movement.
During normal physiological conditions, the time of inspiration is
always greater than the transit time of the slow units. However in pathological
conditions where the respiratory cycle length is decreased especially where
ventilatory strategies such as inverse ratio ventilation or HFOV are used, interregional
to-and-fro mixing (Pendelluft) may
positively influence gas transport. In these circumstances, the sum of the
individual regional expansions is much greater than the VT delivered,
indicating that parenchymal VT changes are enhanced by re-circulating
gases.
Augmented Dispersion (Taylor Dispersion)
The concept of gas dispersion was first described by Taylor in 1953
[3,4]:
Gas dispersion results from the interaction
between the axial velocity profile and radial diffusion of gases in motion.
In the lungs, the branching network of the airways together with Pendelluft movement, leads to gas
turbulence and mixing between the core and the periphery of the gas column and
radial dispersion is eliminated. This results in greater gas mixing with lower
tidal volumes.
Asymmetric
Velocity Profiles
Axial gas velocity profiles in branching systems, such as that
encountered in the tracheobronchial tree have been studied. Under such
conditions, the inspiratory profile is more skewed than the expiratory profile.
Turbulence, accentuated velocities, eddies and swirls of gas all occur at the
bifurcations in the airways which results in greater gas dispersion. At higher
frequencies, inertial effects become more marked and the velocity profiles are
more exaggerated. The net effect is, that even though bulk axial flow is low
with ventilatory modes such as HFOV, the altered velocity profiles and
interactions which occur in a branching system, result in greater gas
dispersion.
Molecular
Diffusion
Molecular diffusion results from the random motion of gas molecules. Gas
transport across the alveolar membrane occurs via this mechanism. The diffusion
gradient generated in the alveolus leads to transport of oxygen (O2)
from the alveolus (high O2) to the pulmonary circulation (low O2)and
removal of CO2 by a diffusion gradient in the opposite direction.
When O2 passes from the alveolus into the pulmonary circulation, the
drop in alveolar O2 provokes a similar diffusion gradient between
the mouth and the alveolus. Diffusion is a slow process and while HFOV may
enhance molecular diffusion, this theory of gas exchange has not been shown to
be more important during HFOV than it is during conventional ventilatory modes.
In
Summary
Overall, effective ventilation using HFOV involves all of the five gas
transport mechanisms described. The different mechanisms may have greater
bearing at different levels of the tracheobronchial tree (Fig 1). The overall coefficient of gas transport during HFOV has
been shown to be a function of the product of VT2 and
frequency (f). [5,6]
1. 
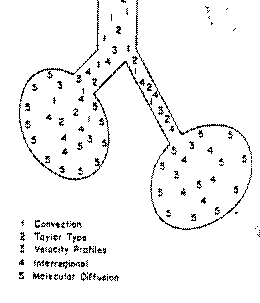
Figure 1: Different mechanisms of gas
transport.1= convection; 2= Taylor dispersion; 3= Velocity profiles; 4=
Interregional and 5 = Molecular diffusion. (Taken from Wetzel RC, Gioia FR.
High frequency Ventilation. Pediatr Clin North America 1987 Feb;34(1):15-38.)
Mechanisms of Acute Lung Injury (ALI)
The ongoing pathological process which occurs in ARDS patients has been
extensively studied. Ventilator patterns have been shown to influence the
extent of acute lung injury [7].The stress invoked by the shear forces exerted
on healthy overdistended alveolar units, during the cyclic alveolar/airway
expansion and collapse which occurs during conventional ventilatory modes
(CMV), is thought to be one of the most important factors in the development of
hyaline membrane disease. The presence of polymorphonuclear neutrophils (PMNs)
in ARDS is a well documented phenomenon. [8] PMNs appear to have a role in this
process, particularly when activated. Masatoshi and co-workers studied
neutrophil influx and activation in surfactant-depleted rabbit lungs (i.e
atelectasis-prone), while applying different ventilator patterns [8]. They
showed that when CMV’s were used an influx of PMNs occurred which became
functionally activated and developed chemotactic properties. The lungs in these
animals went on to develop the characteristic reduced gas exchange and marked
pressure-volume (P-V) curve abnormalities seen in ARDS. In comparison, the
animals ventilated using HFOV had a similar influx of PMNs but functional
activation was not present and progressive deterioration in gas exchange and
V-P curves did not occur. The study showed that prevention of the cyclic
alveolar/airway expansion and collapse in surfactant-deficient lungs minimised
activation of PMNs and subsequent development of ALI.
The low tidal volumes applied during HFOV prevent overdistension of the
few healthy alveolar units. This results in a lower level of shear forces developing in the healthy
units and consequently a lower associated immunological cascade. The study by
Masatoshi et al further suggests that application of such a ventilatory mode
earlier, rather than later (as a rescue technique), would lead to less
pulmonary damage in ARDS patients.
HFOV was first used in neonates suffering from ARDS. High ‰AW are initially applied together with HFOV, as a lung volume optimisation
manoeuvre, in order to actively recruit and maintain open, alveoli, in
atelectatic lung regions. Possible haemodynamic compromise, as a result of such
high ‰AW, was investigated by Gutierrez and co-workers [9]. They studied the
haemodynamic profile via pulmonary artery catheters, in neonates suffering from
ARDS, ventilated with HFOV. They found that although the initial ‰AW was higher than that applied when using CMV, no adverse haemodynamic
effects occurred. Pressure transmission in non-compliant lungs, where high
resistance exists, appears to behave differently to pressure transmission in
normal lungs. [10] Less pressure is transmitted to intrathoracic,
extrapulmonary structures and haemodynamic compromise has not been a problem.
[9, 11]
Use of this technique in the paediatric area indicated that, early
application significantly improved outcome and reduced the number of chronic
complications in ARDS survivors.[12,13] Development of more powerful HFO
ventilators with the capacity to
ventilate adults, lead to a study by Fort et al in 1997 in which HFOV, together
with a lung volume optimisation protocol, was used in adults suffering from
ARDS. [11] The investigators applied a lung volume recruitment strategy which
involved incremental increases in ‰AW to achieve a PaO2 of ³ 60 torr, with an FiO2 of £ 0.6. The study showed that gas exchange
improved significantly in the majority of patients as well as the Pao2/FiO2
ratio (p < 0.02). No significant compromise in cardiac output or
oxygen delivery (DO2) was observed despite significant increases in ‰AW (31.2 ± 10.3 to 34.0 ± 6.7 cm H2O. p < 0.05).
The accompanying editorial [14] of this article describes the advances
in the development of this ventilatory mode
since the original concept of High Velocity Jet Ventilation was first
introduced sixteen years prior to HFOV. The negative effect of CMV, which have
resulted in repeated overdistension of healthy lung regions and ongoing
atelectasis leading to worsening of the underlying pathophysiological process,
is clearly stated. The need to apply
practically, what is now known experimentally, namely the application of an as even as possible expansion pattern in
the atelectasis-prone lung, could improve outcome in this serious condition.
HFOV together with high ‰AW, offers a means of keeping below the upper
inflection point of the P-V curve (where alveolar overdistension occurs), without
haemodynamic compromise. The separation of O2 delivery from CO2
elimination removes the associated problem of hypercarbia.
Figure
2: HFOV ventilator
![]()
Figure
3: Patient with ARDS ventilated using HFOV
Conclusion
In conclusion, HFOV is still a new technique
at present. It was originally studied using surfactant depleted rabbit models
and then applied in paediatric neonatal patients with ARDS. It is currently
being used in adults. A number of studies are underway which should clarify its
role, as one of the treatment options available, for patients with ARDS and low
lung compliance pathologies.
Acknowledgements
I would like to thank the staff, in particular my tutor Dr. Angela
McLuckie, of the Guys and St. Thomas’s Hospital Trust Intensive Care Unit, for
the theoretical and practical guidance offered to me concerning HFOV during a
three-month ICU placement .
Bibliography
2. Wetzel
RC, Gioia FR. High frequency Ventilation. Pediatr Clin North America 1987
Feb;34(1):15-38.
3. Arthur
B. Otis et al. Mechanical Factors in
Distribution of Pulmonary Ventilation. J.Appl Physiol 8:427-433,1956.
4. Taylor
G I. Dispersion of soluble matter in
solvent flowing slowly through a tube. Proc R Soc London 219: 186-203, 1953.
5. Taylor G I. The dispersion of matter in turbulent
flow through a pipe. Proc R Soc London 223:446-468,1954.
6. Carlon
GC, Ray C Miodownik S et al. Physiologic implications of high- frequency jet
ventilation techniques. Crit Care Med 11:508-514,1983.
7. Chang
H K: Mechanisms of gas transport during ventilation by high-frequency
oscillation. J.Appl Physiol 556:553-563,1984.
8. Pamela
R. McCulloch, P. Gek Forkert and Alison B.Froese. Lung Volume Maintenance
prevents Lung Injury during High-frequency oscillatory ventilation in Surfactant-deficient
Rabbits. Am
Ver Resp Dis 1988; 137:1185- 1192.
9. Masatoshi Sugiura et
al. Ventilator pattern influences neutrophil influx and activation in
atelectasis-prone rabbit lung. The American Physiological Society 1994
1355-1365.
10. J A Gutierrez, D L Levin and
L O Toro-Figuerosa. Hemodynamic effects of high-frequency
oscillatory ventilation in severe pediatric respiratory failure. Intensive Care
Med (1995) 21:505-510.
11. Dale
R. Gerstmann, Janie M. Fouke, Dean C. Winter et al. Proximal, Tracheal and Alveolar pressures during
High-frequency oscillatory
ventilation in a Normal Rabbit model. Paediatric Research 1990 Vol. 28
Nº 4: 367-373.
12. Peter
Fort MD et al. High-frequency oscillatory ventilation for adult
respiratory distress syndrome-a pilot study. Crit Care
Med 1997 Vol.25,
Nº6.
13. John H. Arnold,MD; James H.
Hanson,MD; Luis O. Toro-Figueiro et al.
Prospective, randomized comparison of
high-frequency oscillatory
ventilation and conventional mechanical ventilation in pediatric respiratory
failure. Crit. Care Med 1994 Vol. 22, Nº 10: 1530-1539.
14. Reese
H. Clarke,MD; Dale R. Gerstmann,MD; Donald M. Null,MD et al. Pulmonary
Interstistitial emphysema treated by High-frequency oscillatory ventilation.
Crit. Care Med. 1986 Vol.14: 926-939.
15. Alison
B.Froese,MD,FRCP.High-frequency oscillatory ventilation for adult respiratory
distress syndrome : Let´s get it right this time! Crit. Care Med 1997 Vol. 25,
Nº 6: 907-908.
![]()
![]()