PANEL DE DISCUSION |
Manuel Angoso de Guzman
Clinica Virgen del Consuelo mrjnefro@vlc.servicom.es
INTRODUCTION
Most Nephrologists will rely on the surgeon decision on the type of dialysis vascular access their ESRD patients are going to get and then complain if the access is not functioning well or is placed in an anatomic position that is difficult to access by the nurse or uncomfortable for the patient. Furthermore the outcome of the vascular access is dependent of the surgeon interest in hemodialysis access procedures.
The purpose of this article is to give an overview of the preoperative planning process and follow up care that should be performed in a patient with a native arteriovenous fistula.
One major cause of an early arteriovenous fistula ( AVF) failure is the selection of a suboptimal vein and artery.
A simple way to improve the patency rates and decrease the numbers of procedures second and third AVF´s or the use of grafts is to do an adequate evaluation on your patients in collaboration with the vascular surgeon (Table I ).
The initial evaluation should be done when the patient is seen in the Nephrologist office early in the course of disease 1 and not just a few weeks before starting chronic replacement therapy. The time of access placement correlates with access outcomes and with patients mortality2. The mortality risk of those patients who had their access placed 30 days before hemodialysis was double when compared with those who had the access placed 6 months before starting hemodialysis. An early AVF construction should be considered in those patients with a Creatinine >4 mg/dl ( or when Creatinine Clearance approaches 25 ml/min ) unless there is a planned living related transplantation 3. The access will have enough time to develop and the use of central lines will be avoided. Patients with a poor vascular system should be referred, if there are no major contraindications, to a PD program.
The patient should have a complete history of the potential factors that may complicate the creation of an AVF (see Table I )4 which includes a record of all the venipunctures and procedures involving the upper and lower extremities. The hospital staff (venipuncture teams, nurses etc. ) should have instructions on how to manage these patients to avoid unnecessary use of upper arms veins and patients should be thought to preserve integrity of their veins by refusing needle stiks by lab and nursing personel.
| Venipuncture and Arterial lines |
| History of previous thrombosis |
| Central Venous Catheter |
| Surgical History with emphasis upper ext, neck and chest surgery |
| Iodine Allergies |
PHYSICAL EXAMINATION
The physical examination should be done in a warm room after explaining in detail the purpose of the examination to the patient. The arm can be placed in a bowl with warm water to induce venodilatation.
INSPECTION
Inspect both arms from the fingertips up to the shoulders assessing:
-Size and symmetry
-Colour and texture of the skin and nail beds
-Edema
-Any scars from previous surgery
-Presence of collateral circulation in the forearm and shoulder
PALPATION
Arterial Examination
Check the radial pulse in the flexor area of the wrist and compare the volume on each arm. Do the same with the Ulnar Artery (medial in the flexor surface of the wrist) and the brachial Artery (between the biceps and triceps muscle)
According to the physical examination the pulse can be classified:
Absent
Marked diminish
Moderately Diminish
Slight Impaired
Normal
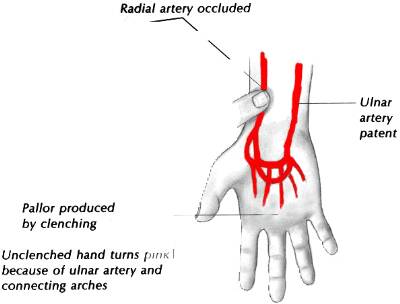
ALLEN TEST
The normal ulnar artery is difficult to palpate, another way to check for patency is to perform the Allen Test.
The patient rests the hand in his lap clenching firmly his fist. The radial Artery is compressed which produces a
decrease in perfusion turning the hand pale. The patient will then open his hand and the skin will recover its
normal pink appearance if the Ulnar artery is patent. Do the same process is done with the ulnar artery (see Fig.1)
Fig 1 Allen Test

Venous Examination
The tourniquet is placed on the upper portion of the arm. Ask the patient to close and open repeatedly the hand
toincrease vein engorgement. The cephalic and basilic vein in the arm, antecubital area and forearm should be
assessed. Raise the upper limb and evaluate venous drainage observing a delay suggesting obstruction.

FIG 2 Arterio-venous anatomy upper extremities
PREOPERATIVE ASSESMENT
ULTRASOUND EXAMINATION
Preoperative mapping of the upper limb vascular tree will increase the patency rate of AVF and decrease the rate of early failure. 5 6This procedure can be done, using a portable ultrasound device to assess:
-the diameter and compressibility of the vein
-The diameter of the radial artery ( a powerful predictor of early fistula dysfunction and failure to mature) 7
We have been doing vein mapping using the Site RiteR (r) (see Table 2 ). The technique is very similar to the physical examination described previously and takes 15-20 min to exam both arms.
The patient is placed lying flat with his arm hanging on the side of the bed

Fig 3 Antecubital venous anatomy
The following criteria modified from Silva et al are used to assess patients' upper extremities' vein adequacy with a portable ultrasound (see Table 2):
Table 2
Venous Examination
Arterial Examination
PROCEDURE OF CHOICE IN VASCULAR ACCESS SURGERY
We should consider the following general guidelines before access placement
-Select the non dominant arm ( if possible )
-Place the access distally to preserve proximal sites
-Avoid atherosclerotic arteries
-The selected veins should have a long segment to allow for variation in puncture sites.
-Those patients with a small and atherosclerotic radial artery should have the access placed over the elbow.
-Try to use the non dominant arm exhausting all reasonable possibilities before going to the other extremity.
Table 3
| Radiocephalic Fistulas
Brescia-Cimino (Wrist)* Snuff-Box 8 |
| Ulnar Cephalic Fistula |
| Forearm Vein Transposition 9 |
| Proximal Forearm Fistula 19
Proximal Radial -Median cephalic Vein Brachial -Cephalic Vein ** Brachial-antecubital Vein
|
| Upper Arm 10
Transposed Basilic Vein 11,12 13 Brachiocephalic Jump
|
| Popliteal -Saphenous Vein*** |
* The anatomical snuffbox median primary patency was 36 months vs 64 months at the wrist 8. For that reason we do not recommend this procedure in our patients although others authors have published excellent results using this technique 14,15
**The diabetic is not considered a good candidate for primary native AVF making the PTFE graft the access of choice for most of these patients but this idea is challenged by recent data. Hakaim et al showed that the cumulative primary patency at 18 months was 33% for a radiocephalic fistula and 78% for a brachiocephalic AVF.The diabetic patient ( Type I/II ) with unsuitable arterio -venous vessels in the wrist, the best procedure should be either a primary braquiocepahalic or a transposed brachio-basilic fistulae
***We do not use saphenous vein transposition in the thigh due to the high incidence of cardiovascular morbidity in patients with chronic renal failure However ,this option may be a consideration in those patients with AIDS 16 or before performing a thigh graft 12,17 .
POSTOPERATIVE CARE
This is our current immediate postoperative care protocol
1-The patient should be monitor and the new AVF evaluated by a Nephrologist /HD nurse
2-Vital Signs check every 30 min. Call the nephrologist on duty if :
BP Systolic < 100 mmHg Diastolic < 60 mmHg, Pulse <60>110 Temp > 38
3-Examination
Inspection : Presence of a haematoma
Palpation : Thrill
Auscultation :Measure the maximum distance that the murmur is heard in the arm
4-If stable discharge the patient after 4 hours
5-The patient will receive instructions to call us or to return to the hospital:
Bleeding from the AVF
Non a palpable thrill
Note :The established practice of asking the patient to do hand exercise after a radiocephalic Fistula is not supported by access blood flow measurement. Exercise does not increase access blood flow 18. However it does increase patients' awareness in the importance of caring for his access .
INTRODUCTION
The creation of an Arterio-Venous Fistula will cause arterial blood ( high speed laminal flow ) to enter the venous low resistance system creating a laminar flow with a continuous turbulence with a systolic peak at the site of the anastomosis -the area with the highest pressure gradient. These ingredients, the increase in pressure and flow in the venous side combined with the spectacular increase in blood flow in the artery, are needed for successful AVF maturation Note: Blood flow in a normal brachial artery is 85 ml/min and will increase by a factor of 5-10 after the fistula is open. The Arterial flow is dependent in a nonlinear relationship with the cross sectional area of the fistula.
We will wait for at least 4 weeks beforet using the new access. During first two or 3 treatments we use a single needle hemodialysis with no heparin. The hand of the patient is secure with a tape to avoid involuntary movement of the arm and trauma to the vein wall by needle motion. Before accessing the AVF, the nurse does a quick evaluation of the access. We use an ultrasound guided needle stick in those accesses that are difficult to stick, i.e. a brachio-basilic vein or an obese patient.
Most stenosis will occur in the efferent vein near the arterial puncture. Stenosis may occurs as early as 2 weeks after access placement and they are responsible of early access failure 7 (see below) .
Access monitoring combined with early access intervention will increases significantly cumulative access patency 20 . There are several methods to screen AVF for signs of early dysfunctions. Most of these methods will detect functional changes in the access rather than anatomical abnormalities.
Functional methods are used to evaluate access dysfunctions and are appropriate for periodic access monitoring while angiography (gold Standard ) and ultrasounds are used for anatomical examination of the access. The AVF can either fail to mature for needle cannulation or later become dysfunctional
FAILURE OF AVF TO MATURE
If after 8 weeks the AVF fails to mature, i.e. BFR < 350 ml/min and/or a recirculation higher than 10% a fistulogram should be done . The ultrasound, being very sensitive and specific for detecting graft dysfunctions, is less reliable for the evaluation of AVF anatomy.(see below)
Beathard et al have recently published their results on salvage of early nonfunctional fistulas. Out of 63 patients with inadequate AVF development 74.7 % had their access patent after 1 year using the following techniques after angiography in a stepwise fashion: percutaneous angioplasty, accessory vein ligation, medial vein ligation and mainstream banding 21. Therefore most AVF that failed to developed should be evaluated since most early AV access dysfunctions can be rescued and become functional sparing the patient the frustration of having to go through the creation of another access and the placement of a central vein line.
AVF MONITORING (see DOQI Guidelines )
There are different methods to assess periodically the AVF access.
PHYSICAL EXAMINATION
VENOUS PRESSURE
Dynamic
Static
RECIRCULATION STUDIES.
Chemical
Dilutional
INTRA-ACCESS BLOOD FLOW MONITORING
Hemodynamic access
Ultrasound dilution technique
Colorflow Duplex
ACCESS ANATOMY
Colorflow Dupplex
Fistulogram
MRI
PHYSICAL EXAMINATION
Although underused and very dependent on the experience and interest of the examiner, a thorough examination of the AVF ( which takes 3-5 min ) will provide much information about access function . At examination there are different clues that can give an idea of the anatomical abnormalities present in the fistula. (See Table 4)
Table 4 The goal , nevertheless , is to be able to pick early those accesses that are going to fail so an elective solution can be
offer avoiding the risk of a long standing central line,the loss of a very valuable venous area and the need to create
another access in a group of patients that cannot afford it. VENOUS PRESSURE 22 Useless for monitoring AVF 23 since, as the intravenous pressure rises, blood flow is diverted through vein collaterals
preventing an increase in venous pressure.Once there is a suspicion of venous stenosis the patient should probably
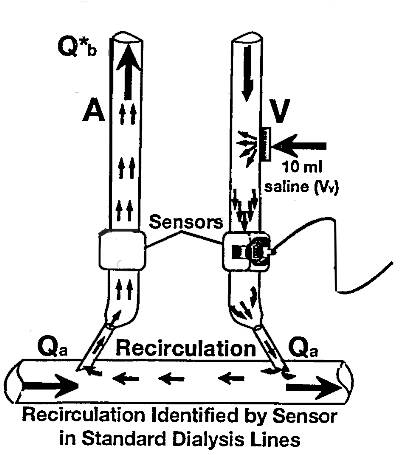
have a fistulogram to assess anatomy. RECIRCULATION Access recirculation (RC) develops when dialyzed blood returning the patient through the venous side reenters the
extracorporeal circuit through the arterial needle. Recirculation decreases significantly the amount of HD delivered
and is a marker for access stenosis. Recirculation occurs when the AVF blood flow rate ( average AVF blood flow
1000 ml /min ) 24 is less than the blood pump. There are several systems to measure RC I will briefly discuss the 2 most common techniques: Urea (or chemical) and ultrasound (or dilutional-based
methods.) PERIPHERAL BLOOD Measure Urea/BUN in a peripheral vein (P) and simultaneously in the Arterial (A) and venous line (V). The percentage of recirculated blood is calculated as (P-A/P-V)x 100. This is the less reliable method to calculate recirculation due to a decrease of Urea in the arterial line ( secondary to
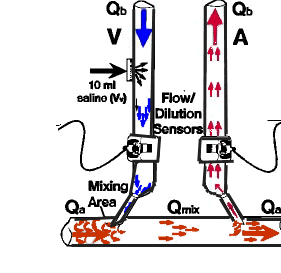
cardiopulmonary recirculation ) 25 and increase in peripheral blood due to difference in regional blood flow 26 LOW FLOW METHOD (DOQI Guidelines ) 27 Draw samples from the arterial (A) and venous (V) line Reduce blood flow rate(BFR) to 120 ml/min Turn blood pump off 10 seconds after reducing BFR Draw systemic (P) arterial sample from the arterial line TRANSONIC SYSTEM The system used intrasonic transit flow measurement with a flow/dilution sensor connected around the arterial and
venous line. The Transonic measure's dialyzer blood flow, access flow and recirculation. Patient evaluation using
the Transonic System (1 recirculation measurement and 2 access flow) requires about 12 minutes. DIALYZER BLOOD FLOW (Qb) The sensor emits an ultrasound beam which will cross the line in both direction. Qb is measured as the difference
between the upstream and downstream transit time ultrasound (see Fig. 4) The Ultrasound measurement of blood velocity is dependent on the protein concentration, the greater the
concentration of protein in the blood the faster will the ultrasound beams travel through it. The administration of
isotonic solution will dilute protein concentration and decrease the ultrasound velocity. By injecting Normal Saline
into the venous port the sensor measure this decrease in ultrasound velocity and reflected as a curve -the dilutional
curve - If recirculation is present, normal saline will be taken by the arterial line, decreasing ultrasound
measured blood velocity-and generating a second dilutional curve. Recirculation is calculated as the ratio of the area
under the arterial curve to the area under the venous curve. (See Fig 5 ) Fig 5 and Fig 6 The lines are reversed and normal saline is injected in the venous side (see Fig. 6 ) Access Flow is calculated from
the ratio of the venous area to the arterial area times the dialyzed blood flow. Access flow measurement should be
done during the first 90 min of treatment and avoided if there is a 10% or more change in MAP 28 . Neyra et al 29
has shown that a time dependent decline in vascular access blood flow is highly predictive of access thrombosis.
Using the transonic system they measure the access blood flow rate in 95 patients ( 23 with native AVF and 72 with
Grafts ). A decline in baseline blood flow rates of more than 15% was associated with a relative risk (RR) of
thrombosis of 4.4.The RR increased to 34 when the decline of BFR was above 50%. The study was limited by the
small number of thrombosis ( 4) and patients with AVF . Nevertheless, emphasis the importance of doing
sequential evaluation of the access rather than looking to an absolute number to assess for early vascular
dysfunction. Fig.7 HEMODYNAMIC RECIRCULATION MONITOR (HDM) The HDM is a non invasive electronic device that uses magnetic principles to measure difference in conductivity
between the arterial and venous side. Lindsay et 30,31 al has shown that the HDM is very accurate measuring access
recirculation with excellent repeatability. ACCESS ANATOMY ULTRASOUND Most of the data available on Doppler ultrasound for evaluation of access dysfunction has been done in grafts.
Gadallah et al 32 evaluated 38 unselected patients with doppler US followed by digital substraction angiography
.They found a good correlation in diagnosing access stenosis between Doppler US and fistulography. There were 13
patients with a native AVF and only 3 had a significant stenosis.The result on the ultrasound evaluation is very
dependant on the experience and interest of the operator. Possible sources of error: -Measurement of the vessel cross section -Poor Doppler wave forms related to an inadequate angle (keep the angle less than 60 ) -Different measures of flow volume calculation FISTULOGRAPHY Venous Fistulography is the gold standard for evaluating AVF anatomy .It is simple, very accurate and with a low
complication rate and is the procedure of choice for preoperative evaluation of a malfunctioning access although
should not be used for routine AVF screening. The venous angiography will outline: -the size and type of Fistula -Size, shape and position of the veins used for cannulation -Presence of aneurysm and/or thrombi, -Locations of venous stenosis MRI33 The AVF anatomy can be visualized in those patients who are allergic to iodine contrast with MR -imaging. The
MRI is a non invasive procedure that offers 2 methods for evaluating AVF: -PHASE CONTRAST MRI: measure flow rates and gives an idea of the AVF access anatomy but it will
overestimate stenosis due to motion artefacts induced by the post stenosis turbulent blood flow. -CONTRAST ENHANCED MRI: Will define the venous anatomy with precision but would not give any
information about access flow and the measurement is done in the axial plane. PERCUTANEOUS TRANSLUMINAL ANGIOPLASTY (PTA ) 34 Most of the data on PTA comes from the radiology literature and applies to the treatment of the failing graft. The
advantages of PTA are numerous (see Table 5) .The results on PTA access patency available on the radiology
literature are difficulty to compare with the results obtained from surgical literature. For instances primary
patency in the radiology literature is expressed after pta access dilatation while in the surgical literature the data is
expressed as either primary patency ( creation of the access until the surgical intervention ) or cumulative patency
(as the access patency regardless of the number of procedures done on that access).Therefore is complicated to
compare the different series since there are being expressed in a different way . Table 5 summarized the results
published in the literature . Table 5 Vein preservation Avoids Temporary Access High Technical Success Repeated PTA Risk of Arterial Steel Syndrome in upper arm access 82%
The cumulative patency of PTA on AVF is excellent and comparable to the results obtain with surgical revision
but with the added benefit of vein preservation and minimal invasive technique. This result emphasizes the
important role that the interventional radiologist or nephrologist have in management of AVF complications and in
increasing the life span of the AVF. COMPLICATIONS CARDIAC FAILURE ( High Output) 29 39 High output cardiac failure is a rare complication occurring more frequently in patients with a braquiocephalic or
transposed basilic vein access. The treatment is either banding or ligation of the access. Banding can be complicated
with venous hypertension and the development of an upper arm edema. NEUROPATHY This complication ,Ischemic monomelic neuropathy, is seen in patients with peripheral vascular disease -most
frequently in diabetics.The patient developed a severe excruciating pain of the involved extremity with wrist drop
suggestive of radial injury at the levels of the elbow secondary to peripheral ischemic neuropathy 40-42 .Treatment
consist in fistula ligation . VENOUS HYPERTENSION 43-45 The hand distal to the access is swollen with thickening and hyperpigmentation of the skin .In extreme cases there
is distal extremities ulceration or the development of a kaposi -like sarcoma46.The use of central Vein lines may lead
to central vein stenosis or thrombosis with the development of massive upper extremity edema after access
placement . STEEL SYNDROME 47,4849,50 Some degree of retrograde flow occurs in almost every AVF being higher in side-to side anastomosis and in
braquiocephalic fistula .For example in a normal radiocephalic fistula 20 % of blood will come from the distal
artery .(Anderson ) The steel syndrome occurs due to a retrograde flow from the distal artery of the anastomosis
to the low resistance venous system causing the blood flow tol go from the ulnar to the radial artery through the
palmar arch with hypoperfusion of the palm and forefingers .The incidence is higher in the artherosclerotic and
diabetic patients . The clinical presentation is a painful, cold and clammy hand that gets worst on hemodialysisIt
can lead to acral gangrene and finger amputation . To prevent this problem in high risk patient distal radial artery
ligation can be performed . End-to-end anastomosis is complicated by technical problems like vessel rotation
during the anastomosis or higher percentage of stenosis which will increse the risk of poor blood flow rate and
early thrombosis . ACKNOWLEDGEMENT I am indebted to Vo Nguyen MD ( Memorial Clinic Olympia, WA ) for his critical review and comments of this
manuscript . REFERENCE 1. Stehman-Breen CO, Sherrard DJ, Gillen D, Caps M. Determinants of type and timing of initial permanent
hemodialysis vascular access. Kidney Int 2000;57(2):639-45. 2. Chesser AM, Baker LR. Temporary vascular access for first dialysis is common, undesirable and usually
avoidable. Clin Nephrol 1999;51(4):228-32. 3. Hakim R, Himmelfarb J. Hemodialysis access failure: a call to action. Kidney Int 1998;54(4):1029-40. 4. De Marchi S, Falleti E, Giacomello R, et al. Risk factors for vascular disease and arteriovenous fistula dysfunction
in hemodialysis patients. J Am Soc Nephrol 1996;7(8):1169-77. 5. Silva MB, Jr., Hobson RW, 2nd, Pappas PJ, et al. A strategy for increasing use of autogenous hemodialysis access
procedures: impact of preoperative noninvasive evaluation. J Vasc Surg 1998;27(2):302-7; discussion 307-8. 6. Ascher E, Gade P, Hingorani A, et al. Changes in the practice of angioaccess surgery: Impact of dialysis outcome
and quality initiative recommendations. J Vasc Surg 2000;31(1):84-92. 7. Wong V, Ward R, Taylor J, Selvakumar S, How TV, Bakran A. Factors associated with early failure of
arteriovenous fistulae for haemodialysis access. Eur J Vasc Endovasc Surg 1996;12(2):207-13. 8. Simoni G, Bonalumi U, Civalleri D, Decian F, Bartoli FG. End-to-end arteriovenous fistula for chronic
haemodialysis: 11 years' experience. Cardiovasc Surg 1994;2(1):63-6. 9. Silva MB, Jr., Hobson RW, 2nd, Pappas PJ, et al. Vein transposition in the forearm for autogenous hemodialysis
access. J Vasc Surg 1997;26(6):981-6; discussion 987-8. 10. Gade J, Aabech J, Hansen RI. The upper arm arterio-venous fistula--an alternative for vascular access in
haemodialysis. Scand J Urol Nephrol 1995;29(2):121-4. 11. Coburn MC, Carney WI, Jr. Comparison of basilic vein and polytetrafluoroethylene for brachial arteriovenous
fistula. J Vasc Surg 1994;20(6):896-902; discussion 903-4. 12. Gaudiani VA, Plecha FR. A technique for the placement of popliteal artery to saphenous vein prosthetic grafts
for hemodialysis access. Surg Gynecol Obstet 1980;150(5):729-31. 13. Humphries AL, Jr., Colborn GL, Wynn JJ. Elevated basilic vein arteriovenous fistula. Am J Surg
1999;177(6):489-91. 14. Bonalumi U, Civalleri D, Rovida S, Adami GF, Gianetta E, Griffanti-Bartoli F. Nine years' experience with
end-to-end arteriovenous fistula at the 'anatomical snuffbox' for maintenance haemodialysis. Br J Surg
1982;69(8):486-8. 15. Boccardo G, Ettari G, Donato G, De Prisco O, Maurino D. [High-flux arteriovenous fistula at the anatomic
snuffbox]. Minerva Urol Nefrol 1998;50(1):39-43. 16. Gorski TF, Nguyen HQ, Gorski YC, Chung HJ, Jamal A, Muney J. Lower-extremity saphenous vein
transposition arteriovenous fistula: an alternative for hemodialysis access in AIDS patients. Am Surg
1998;64(4):338-40. 17. May J, Harris J, Fletcher J. Long-term results of saphenous vein graft arteriovenous fistulas. Am J Surg
1980;140(3):387-90. 18. Rodriguez Moran M, Almazan Enriquez A, Ramos Boyero M, Rodriguez Rodriguez JM, Gomez Alonso A.
Hand exercise effect in maturation and blood flow of dialysis arteriovenous fistulas ultrasound study. Angiology
1984;35(10):641-4. 19. Gordon IL. VASCULAR ACCESS Principles and Practice.Ed Wilson SE. 20. Yasuhara H, Shigematsu H, Muto T. Results of arteriovenous fistula revision in the forearm. Am J Surg
1997;174(1):83-6. 21. Beathard GA, Settle SM, Shields MW. Salvage of the nonfunctioning arteriovenous fistula [see comments]. Am
J Kidney Dis 1999;33(5):910-6. 22. Mahmutyazicioglu K, Kesenci M, Fitoz S, Buyukberber S, Sencan O, Erden I. Hemodynamic changes in the
early phase of artificially created arteriovenous fistula: color Doppler ultrasonographic findings. J Ultrasound Med
1997;16(12):813-7. 23. Besarab A, Lubkowski T, Frinak S, Ramanathan S, Escobar F. Detecting vascular access dysfunction. Asaio J
1997;43(5):M539-43. 24. Besarab A, Lubkowski T, Frinak S, Ramanathan S, Escobar F. Detection of access strictures and outlet stenoses
in vascular accesses. Which test is best? Asaio J 1997;43(5):M543-7. 25. Schneditz D, Polaschegg HD, Levin NW, et al. Cardiopulmonary recirculation in dialysis. An underrecognized
phenomenon. Asaio J 1992;38(3):M194-6. 26. Van Stone JC. Peripheral venous blood is not the appropriate specimen to determine the amount of
recirculation during hemodialysis. Asaio J 1996;42(1):41-5. 27. NKF-DOQI clinical practice guidelines for vascular access. National Kidney Foundation-Dialysis Outcomes
Quality Initiative. Am J Kidney Dis 1997;30(4 Suppl 3):S150-91. 28. Rehman SU, Pupim LB, Shyr Y, Hakim R, Ikizler TA. Intradialytic serial vascular access flow measurements.
Am J Kidney Dis 1999;34(3):471-7. 29. Neyra NR, Ikizler TA, May RE, et al. Change in access blood flow over time predicts vascular access thrombosis.
Kidney Int 1998;54(5):1714-9. 30. Lindsay RM, Blake PG, Malek P, Posen G, Martin B, Bradfield E. Accuracy and precision of access
recirculation measurements by the hemodynamic recirculation monitor. Am J Kidney Dis 1998;31(2):242-9. 31. Lindsay RM, Bradfield E, Rothera C, Kianfar C, Malek P, Blake PG. A comparison of methods for the
measurement of hemodialysis access recirculation and access blood flow rate. Asaio J 1998;44(1):62-7. 32. Gadallah MF, Paulson WD, Vickers B, Work J. Accuracy of Doppler ultrasound in diagnosing anatomic stenosis
of hemodialysis arteriovenous access as compared with fistulography. Am J Kidney Dis 1998;32(2):273-7. 33. Laissy JP, Menegazzo D, Debray MP, et al. Failing arteriovenous hemodialysis fistulas: assessment with
magnetic resonance angiography. Invest Radiol 1999;34(3):218-24. 34. Sukhanov VA, Nazarov AV, Zlokazov VB, Chuzhinov SV. [The use of thrombolytic preparations for the
functional restoration of the arteriovenous fistula in patients on programmed hemodialysis]. Ter Arkh
1989;61(12):89-91. 35. Barrill G, Gruss E, Tagarro D, Alvarez V, Traver JA. Treatment with expandable endovascular prosthesis
(Palmaz) to resolve an aneurysmal zone in an arteriovenous fistula (AVF) in a haemodialysis patient [letter].
Nephrol Dial Transplant 1994;9(8):1212-3. 36. Castellan L, Miotto D, Savastano S, Chiesura-Corona M, Pravato M, Feltrin GP. [The percutaneous
transluminal angioplasty of Brescia-Cimino arteriovenous fistulae. An evaluation of the results]. Radiol Med
(Torino) 1994;87(1-2):134-40. 37. Bohndorf K, Gladziwa U, Kistler D, et al. [Treatment of stenosed or occluded hemodialysis shunts. Results of
percutaneous angioplasty and combined radiologic-surgical therapy]. Rofo Fortschr Geb Rontgenstr Neuen Bildgeb
Verfahr 1993;158(6):525- 31. 38. Romero A, Polo JR, Garcia Morato E, Garcia Sabrido JL, Quintans A, Ferreiroa JP. Salvage of angioaccess
after late thrombosis of radiocephalic fistulas for hemodialysis. Int Surg 1986;71(2):122-4. 39. Isoda S, Kajiwara H, Kondo J, Matsumoto A. Banding a hemodialysis arteriovenous fistula to decrease blood
flow and resolve high output cardiac failure: report of a case. Surg Today 1994;24(8):734-6. 40. Pirzada NA, Morgenlander JC. Peripheral neuropathy in patients with chronic renal failure. A treatable source
of discomfort and disability. Postgrad Med 1997;102(4):249-50, 255-7, 261. 41. Wilbourn AJ, Furlan AJ, Hulley W, Ruschhaupt W. Ischemic monomelic neuropathy. Neurology
1983;33(4):447-51. 42. Bolton CF, Driedger AA, Lindsay RM. Ischaemic neuropathy in uraemic patients caused by bovine
arteriovenous shunt. J Neurol Neurosurg Psychiatry 1979;42(9):810-4. 43. Kahn D, Pontin AR, Jacobson JE, Matley P, Beningfield S, van Zyl-Smit R. Arteriovenous fistula in the
presence of subclavian vein thrombosis: a serious complication. Br J Surg 1990;77(6):682. 44. Glaze RC, MacDougall ML, Wiegmann TB. Thrombotic arm edema as a complication of subclavian vein
catheterization and arteriovenous fistula formation for hemodialysis. Am J Kidney Dis 1986;7(5):439-41. 45. Stone WJ, Wall MN, Powers TA. Massive upper extremity edema with arteriovenous fistula for hemodialysis. A
complication of previous pacemaker insertion. Nephron 1982;31(2):184-6. 46. Bogaert AM, Vanholder R, De Roose J, et al. Pseudo-Kaposi's sarcoma as a complication of Cimino-Brescia
arteriovenous fistulas in hemodialysis patients. Nephron 1987;46(2):170-3. 47. Halevy A, Halpern Z, Negri M, et al. Pulse oximetry in the evaluation of the painful hand after arteriovenous
fistula creation. J Vasc Surg 1991;14(4):537-9. 48. Lin G, Kais H, Halpern Z, et al. Pulse oxymetry evaluation of oxygen saturation in the upper extremity with an
arteriovenous fistula before and during hemodialysis. Am J Kidney Dis 1997;29(2):230-2. 49. Mactier RA, Stewart WK, Parham DM, Tainsh JA. Acral gangrene attributed to calcific azotaemic arteriopathy
and the steal effect of an arteriovenous fistula. Nephron 1990;54(4):347-50. 50. Kotval PS, Shah PM, Berman H. Doppler diagnosis of subclavian steal due to arteriovenous hemodialysis fistula
in the ipsilateral arm. J Ultrasound Med 1989;8(12):697-700.
NORMAL
ARTERIAL STENOSIS
VENOUS STENOSIS Inspection
vein dilated with no
collaterals
decrease or absence of
postanastomosis vein
dilatation
post-anastomosis
segmental vein dilatation Auscultation
maximum at anastomosis
with distal radiation
Weak with no radiation
Bruit in the anastomosis
site and a second bruit
over the stenotic area Palpation
maximum thrill at the AVF
junction
pulsatile with absence of
thrill
Presence of two thrills
-AVF and in the stenotic
vein segment. Arm elevation
Complete collapse of the
vein's
Complete venous collapse
non collapsible pre stenotic
vein segment with normal
post-stenosis vein collapse CHEMICAL
DILUTIONAL
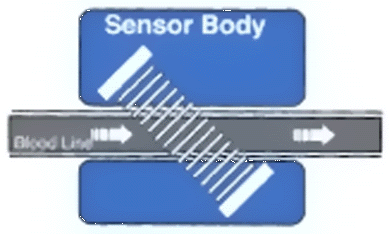
ACCESS RECIRCULATION
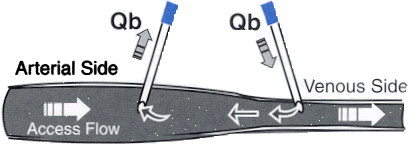
ACCESS FLOW
PERCUTANEOUS ANGIOPLASTY -SUMMARY Advantage
Outpatient procedure
Disadvantage
Not permanent
Author
PRIMARY PATENCY
CUMULATIVE PATENCY
6 months
1 year
2 years
6 months
1 year
2 years Castellan
et al35
79%
61%
61%
90%
83%
83% Bohndorf
et al36
80%
65% *Romero et
al 37
51%
37%
85%
82%
Turmel-Rodrigues et
al 38
Forearm
51%
37%
85%
UpperArm
35%
24%
82%
69%
Except for elastic lesions, which are centrally located , there is little or no use of stenting a proximal stenosis. The
stent itself is going to induce father hyperplasia with post-stent stenosis and early dysfunctions of the access.