In progressive renal diseases the early insult to the kidney depends on an hemodynamic adaptation of remaining units presumably triggered by focal obliteration of most nephrons affected by the original disease (13). Over time glomerulosclerosis and tubule atrophy develop further reducing renal mass in a self-perpetuating process of tissue injury which normally ends with interstitial fibrosis and progressive renal dysfunction. The renal community has discussed for years whether hemodynamic factors were the sole contributors to glomerular and tubulointerstitial injury or whether others mechanisms were also operating. Recent studies have suggested that proteinuria, previously considered just a marker of the severity of a given disease, may itself be pathogenic (12).
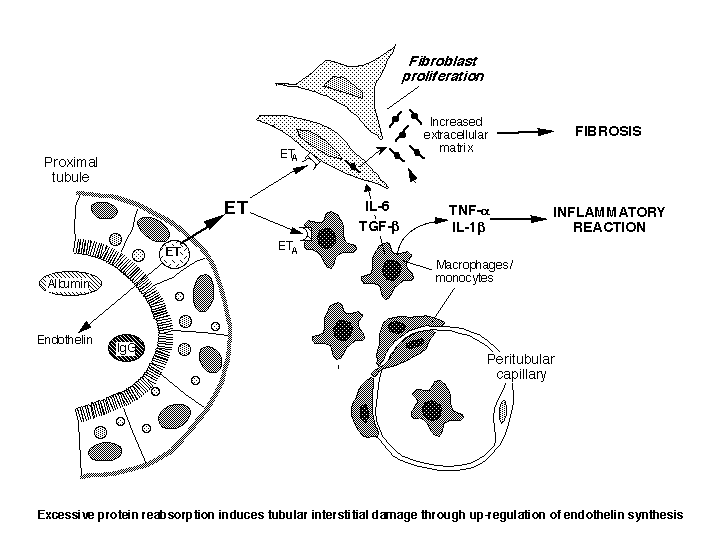
Of interest in this context are findings that the amount of protein recovered in the urine correlated better than any other clinical or laboratory parameters - including the nature of the underlying disease - with the tendency to progress to end stage kidney both in animals and humans. Enhanced protein traffic can cause disease progression through extensive endocytosis of proximal tubular cells that acquire an inflammatory phenotype ultimately responsible of progressive injury of the renal interstitium (Figure). On this line are data that exposure of proximal tubular cells in culture to increasing concentrations of albumin, IgG or transferrin induced a dose-dependent increase in ET-1 production, which was mainly released basolaterally (4). If the same condition would occur in vivo, ET-1 may accumulate in the interstitial compartment and, by binding to specific receptors on interstitial fibroblasts, may induce their proliferation and accumulation of matrix synthesis. A number of studies have convincingly indicated ET-1 as a profibrotic agent for the kidney.
Enhanced renal gene expression and synthesis of ET-1 occurs in vivo in proteinuric progressive renal disease models due to immune or nonimmune damage to the kidney. The remnant kidney model in rats is characterized by a time-dependent increase in renal ET-1 gene expression, paralleled by an increase in urinary excretion of the peptide; both correlating with the degree of interstitial fibrosis ang glomerulosclerosis (14). Gene expression of endothelin receptors in the kidneys of rats undergoing renal mass ablation was differentially modulated during the course of the disease. While ETA receptors were unchanged a selective upregulation of ETB receptors was found, possibly as a consequence of compensatory renal hypertrophy as recently demonstrated in ETB knock-out mice (15). Changes of ET-1 urinary excretion were also observed in rats with passive Heymann nephritis, an immune model of glomerular disease resembling human membranous nephropathy (16). Up-regulation of renal ET-1 and ETB receptor gene expression in NZB/WF1 mice with an immunological disease reminiscent of human lupus as well as in rats after puromycin aminonucleoside injection has been described (17,18). In experimental diabetes mRNA for ET-1 is overexpressed in the kidney in the face of unchanged ETA and ETB receptors (19).
Direct evidence for ET-1 to play a role in progressive renal damage derives from studies with transgenic animals. Transgenic mice for the human ET-1 promoter have increased renal synthesis of ET-1 and develop renal fibrosis and glomerulosclerosis with no changes in systemic blood pressure (20). Furthermore rats transgenic for the human ET-2 gene are normotensive but have renal lesions reminiscent of those of rats with remnant kidney (21).
Effectiveness of ET receptor antagonists to slow or even halt renal disease progression has been consistently reported. In the rat remnant kidney, a specific antagonist for the ETA receptor (22) ameliorates renal functional impairment and protects against glomerular and tubulointerstitial structural injury. An orally active compound with antagonizing properties for ETA and ETB receptors (23) even prolonged animal survival. Similar results have been obtained by other investigators (24). The renoprotective effect of ETA receptor antagonists also was evident in mice with experimental lupus nephritis. In rats with streptozotocin-induced experimental diabetes either if treated at the time of induction of the disease or when animals had already overt proteinuria (25,26).
Manipulation of the endothelin system in the rat by a selective ETA receptor antagonist ameliorated renal dysfunction, prevented renal damage (27), and even prolonged survival in chronic renal allograft nephropathy (28). Of note, ET receptor antagonists, that consistently reduce renal damage, did not invariably normalize proteinuria, strenghtening the hypothesis that excessive ET-1 is not a cause but rather a consequence of increased glomerular protein traffic. Theoretically, the combination of a drug that effectively reduces proteinuria with an endothelin receptor antagonist would have offered a superior renoprotection than single treatments alone. This is actually demonstrated by recent data obtained in a severe model of proteinuric progressive nephropathy not completely responsive to ACE inhibitors in term of reduction of proteinuria, in which combined administration of ACE inhibitor and a selective ET receptor antagonist ameliorated renal function impairment and prevented renal damage better than each drug alone (29).
Evidence for a potential role of ET-1 is also available
in patients with chronic progressive nephropathies, based on plasma and
urine immunoreactive ET-1 measurements (30). Plasma concentration of ET-1
in patients with end-stage renal failure undergoing hemodialysis are one-
to twofold greater than normal (31), possibly as a consequence of an
impairment of clearance. However, the fact that urinary excretion of ET-1
is also increased in patients with chronic glomerulonephritis (30) suggests
that renal generation of ET-1 in these diseases is increased. The recent
finding of a concomitant upregulation of ET-1 and ETB and ECE-1 a gene expression in patients with
glomerulopathy and high proteinuria is highly suggestive of a activation of
renal endothelin system in man predicted by animal studies in progressive
renal diseases (23). Moreover, administration of an ETA/ETB receptor
antagonist to patients with chronic renal failure reduced blood pressure
and renal vascular resistance (32).
In conclusion, pre-clinical observations convincingly
document a role of ET-1 in progressive renal disease and studies with
endothelin receptor antagonists indicate that these compounds, while having
a modest antiproteinuric effect, effectively prevent renal fibrosis.
Clinical studies are now emerging indicating a role for
endothelin also in human progressive nephropathies. Due to the availability
of endothelin receptor antagonists for human use, future clinical studies
are now needed.
1. Yanagisawa M, Kurihara H, Kimura S, Tomobe Y,
Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T: A novel potent
vasoconstrictor peptide produced by vascular endothelial cells. Nature
332:411-415, 1988 2. Inoue A, Yanagisawa M, Kimura S, Kasuya Y,
Miyauchi T, Goto K, Masaki T: The human endothelin family: three structurall
y and pharmacologically distinct isopeptides predicted by three separate
genes. Proc Natl Acad Sci U S A 86:2863-2867, 1989 3. Wagner OF, Christ G, Wojta J, Vierhapper J,
Parzer S, Nowotney PJ, Schneider B, Waldhausl W, Binder B: Polar secretion
of endothelin-1 by cultured endothelial cells. J Biol Chem 267:16066-16068,
1992 4. Zoja C, Morigi M, Figliuzzi M, Bruzzi I, Oldroyd
S, Benigni A, Ronco PM, Remuzzi G: Proximal tubular cell synthesis and
secretion of endothelin-1 on challenge with albumin and other proteins. Am
J Kidney Dis 26:934-941, 1995 5. Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S:
Cloning and expression of a cDNA encoding an endothelin receptor. Nature
348:730-732, 1990 6. Sakurai T, Yanagisawa M, Inoue A, Ryan US, Kimura
S, Mitsui Y, Goto K, Masaki T: cDNA cloning, sequence analysis and tissue
distribution of rat preproendothelin-1 mRNA. Biochem Biophys Res Commun
175:44-47, 1991 7. Benigni A, Remuzzi G: Endothelin antagonists.
Lancet 353:133-138, 1999 8. De Nucci G, Thomas R, D'Orleans-Juste P, Antunes
E, Walder C, Warner TD, Vane JR: Pressor effects of circulating endothelin
are limited by its removal in the pulmonary circulation and by the release
of prostacyclin and endothelium-derived relaxing factor. Proc Natl Acad Sci
USA 85:9797-9800, 1988 9. Sorensen SS, Madsen JK, Pedersen EB: Systemic and
renal effect of intravenous infusion of endothelin-1 in healthy human
volunteers. Am J Physiol 266:F411-F418, 1994 10. Simonson MS, Dunn MJ: Renal actions of endothelin
peptides. Curr Opin Nephrol Hyperten 2:51-60, 1993 11. Achmad T, Rao G: Chemotaxis of human blood
monocytes towards endothelin-1 and the influence of calcium channel
blockers. Biochem Biophys Res Commun 189:994-1000, 1992 12. Remuzzi G, Ruggenenti P, Benigni A: Understanding
the nature of renal disease progression. Kidney Int 51:2-15, 1997 13. Brenner BM, Meyer TW, Hostetter TH: Dietary
protein intake and the progressive nature of kidney disease: The role of
hemodynamically mediated glomerular injury in the pathogenesis of
progressive glomerular sclerosis in aging, renal ablation, and intrinsic
renal disease. N Engl J Med 307:652-659, 1982 14. Orisio S, Benigni A, Bruzzi I, Corna D, Perico N,
Zoja C, Benatti L, Remuzzi G: Renal endothelin gene expression is increased
in remnant kidney and correlates with disease progression. Kidney Int
43:354-358, 1993 15. Liu B, Yanagisawa M, Preisig P: The endothelin B
(ETB) receptor mediates compensatory renal hypertrophy. J Am Soc Nephrol
10:496A, 1999 16. Zoja C, Liu X-H, Abbate M, Corna D, Schiffrin EL,
Remuzzi G, Benigni A: Angiotensin II blockade limits tubular protein
overreabsorption and the consequent up-regulation of endothelin-1 gene in
experimental membranous nephropathy. Exp Nephrol 6:121-131, 1998 17. Nakamura T, Ebihara I, Fukui M, Osada S, Tomino
Y, Masaki T, Goto K, Furuichi Y, Koide H: Renal expression of mRNAs for
endothelin-1, endothelin-3 and endothelin receptors in NZB/W F1 mice. Renal
Physiol Biochem 16:233-243, 1993 18. Nakamura T, Ebihara I, Fukui M, Osada S, Tomino
Y, Masaki T, Goto K, Furuichi Y, Koide H: Modulation of glomerular
endothelin and endothelin receptor gene expression in
aminonucleoside-induced nephrosis. J Am Soc Nephrol 5:1585-1590, 1995 19. Fukui M, Nakamura T, Ebihara I, Osada S, Tomino
Y, Masaki TM, Goto K, Furuichi Y, Koide H: Gene expression for endothelins
and their receptors in glomeruli of diabetic rats. J Lab Clin Med
122:149-156, 1993 20. Hocher B, Thone-Reineke C, Rohmeiss P, Schmager
F, Slowinski T, Burst V, Siegmund F, Quertermous T, Bauer C, Neumayer H-H, S
chleuning W-D, Theuring F: Endothelin-1 transgenic mice develop
glomerulosclerosis, interstitial fibrosis, and renal cysts but not
hypertension. J Clin Invest 99:1380-1389, 1997 21. Liefeldt L, Bocker W, Schonfelder G, Zintz M,
Paul M: Regulation of the endothelin system in transgenic rats expressing
the human endothelin-2 gene. J Cardiovasc Pharmacol 26:S32-S35, 1995 22. Benigni A, Zoja C, Corna D, Orisio S, Longaretti
L, Bertani T, Remuzzi G: A specific endothelin subtype A receptor
antagonist protects against injury in renal disease progression. Kidney Int
44:440-444, 1993 23. Benigni A, Zoja C, Corna D, Orisio S, Facchinetti
D, Benatti L, Remuzzi G: Blocking both type A and B endothelin receptors in
the kidney attenuates renal injury and prolongs survival in rats with
remnant kidney. Am J Kidney Dis 27:416-423, 1996 24. Nabokov A, Amann K, Wagner J, Gehlen F, Munter K,
Ritz E: Influence of specific and non-specific endothelin receptor
antagonists on renal morphology in rats with surgical renal ablation.
Nephrol Dial Transplant 11:514-520, 1996 25. Nakamura T, Ebihara I, Tomino Y, Koide H: Effect
of a specific endothelin A receptor antagonist on murine lupus nephritis.
Kidney Int 47:481-489, 1995 26. Benigni A, Colosio V, Brena C, Bruzzi I, Bertani
T, Remuzzi G: Unselective inhibition of endothelin receptors reduces renal
dysfunction in experimental diabetes. Diabetes 47:450-456, 1998 27. Orth SR, Odoni G, Amann K, Strzelczyk P, Raschack
M, Ritz E: The ETA receptor blocker LU 135252 prevents chronic transplant
nephropathy in the "Fisher to Lewis" model. J Am Soc Nephrol 10:387-391,
1999 28. Rohmeiss P, Braun C, Conzelmann T, Vetter S,
Schaub M, Back WE, Kirchengast M, van der Woude FJ: Improvement of chronic
renal allograft rejection in rats with the oral endothelin A-receptor
antagonist LU135252. J Am Soc Nephrol 9:656A, 1998 (abstract) 29. Benigni A, Corna D, Maffi R, Benedetti G, Zoja C,
Remuzzi G: Renoprotective effect of contemporary blocking of angiotensin II
and endothelin-1 in rats with membranous nephropathy. Kidney Int
54:353-359, 1998 30. Ohta K, Hirata Y, Shichiri M, Kanno K, Emori T,
Tomita K, Marumo F: Urinary excretion of endothelin-1 in normal subjects
and patients with renal disease. Kidney Int 39:307-311, 1991 31. Koyama H, Tabata T, Nishzawa Y, Inoue T, Morii H,
Yamaji T: Plasma endothelin levels in patients with uraemia. Lancet
333:991-992, 1989 32. Webb DJ, Monge JC, Rabelink TJ, Yanagisawa M:
Endothelin: new discoveries and rapid progress in the clinic. Trends
Pharmacol Sci 19:5, 1998
REFERENCES