PANEL DE DISCUSION |
TRANSFORMING GROWTH FACTOR-ß1 AND ANGIOTENSIN II BLOCKADE IN RENAL TRANSPLANT RECIPIENTS.
Josep M. Campistol & Pablo Iñigo,
Renal Transplant Unit, Hospital Clinic, IDIBAPS*,
University of Barcelona, Barcelona, Spain.
Address for correspondence:
-
Dr.Josep M. Campistol
Renal Transplant Unit
170, Villarroel .08036, BARCELONA. SPAIN
Tel. 34.93.227.54.23; FAX 34.93.27.54.98
IDIBAPS*: Institut d’Investigació Biomediques Agusti Pi I Sunyer.
INTRODUCTION
Hypertension represents one of the most common clinical problems in renal transplant recipients, the prevalence ranging from 60 to 85% in transplanted patients treated with Cyclosporine A or Tacrolimus (1,2). Its presence has been closely related to the progressive increase in cardiovascular mortality and morbidity observed in transplanted patients in the lasts years, and to the development and progression of chronic allograft nephropathy (3). Nowadays, cardiovascular mortality is the leading cause of mortality in the renal transplant population, in front of infectious diseases (4). Several factors have been implicated in this dramatic increase in cardiovascular mortality, particularly hypertension, increasing number of diabetic patients, left ventricular hypertrophy and the progressive aging of transplant patients (4,5). Chronic allograft nephropathy is the first cause of graft lost after the first year of functioning kidney, and it represents a complex and multietiological entity in which hypertension has been clearly associated (6).
The etiology of hypertension in renal transplant recipients is multifactorial, and has been associated with several factors, such as previous hypertension, cyclosporine therapy, treatment with steroids, activation of renal renin-angiotensin system, renal insufficiency and, in a minority of patients, renal artery stenosis (1,2). Cyclosporine A therapy is probably one of the main factors responsible for the development of hypertension, since the prevalence of hypertension has increased dramatically with the introduction of cyclosporine (7,8). In the pre-cyclosporine era the prevalence of hypertension was much lower (<40%) AND CARDIOVASCULAR MORTALITY SIGNIFICANTLY LESS (9).
Cyclosporine A induces hypertension through several well known mechanisms: i) it decreases the synthesis of endothelial nitric oxide; ii) it increases the sympathetic tone; iii) it increases the synthesis of endothelin-1; iv) it increases the synthesis of vasoconstrictor prostaglandins; and v) it activates the renal renin-angiotensin system (7-9). Moreover, cyclosporine, through a direct effect and through the overexpression of angiotensin II (AngII), increase the synthesis of transforming growth factor-ß1 (TGF-ß1), which has also been involved in the development of hypertension and mainly in the development and progression of chronic allograft nephropathy (10,11). For these reasons, AngII is a key factor in renal transplant recipients through its decisive role in the development of hypertension, renal insufficiency and cardiovascular mortality (12).
Although theoretically the use of ACE inhibitors and, more recently AngII receptor blocking drugs might be widespread (predominant) in renal transplant recipients with hypertension, its use is restricted to a few Units which have experience with these drugs (13,14). Nevertheless, calcium channel blockers are the group of antihypertensive drugs most frequently used in the treatment of hypertension in renal transplant recipients (15). The reasons for this contradictory situation are diverse: i) the fear of the presence of a renal artery stenosis in the graft, with the risk of an acute renal failure; ii) the slight increases in serum creatinine observed simultaneously to the introduction of an ACE inhibitor or an AngII receptor antagonist always create doubts about an acute graft rejection and are generally missed; and iii) the ease and antihypertensive efficacy of calcium channel blockers therapy in renal transplant recipients, with some beneficial effects on cyclosporine metabolism. Although calcium channel blockers are highly effective as antihypertensive drugs in this population, their tolerance is not as good, with edema and facial flushing in many patients, and no antiproteinuric or renoprotective effect on the graft have been described.
The introduction of AngII receptor antagonists, especially losartan, in the last few years has changed the scenario of hypertension and cardiovascular risk factors in renal transplant recipients; losartan presents high efficacy and excellent tolerance in the treatment of hypertension, proteinuria, chronic graft nephropathy, and also post-transplant erythrocitosis.
Hypertension
In a prospective multicenter Spanish study, we have recently demonstrated the efficacy and safety of losartan in the treatment of hypertension in renal transplant recipients (16). From eight different transplant units, seventy-six renal transplant patients with systolic blood pressure > 140 and/or diastolic blood pressure > 90 mmHg, and/or patients on therapy with one antihypertensive drug and related side effects were recruited. After inclusion, therapy with losartan 50mg/day was started, discontinuing previous antihypertensive therapy, and at four weeks if arterial blood pressure was not controlled, hydrochlorothiazide (25mg/day) was introduced. Mean blood pressure decreased from 113 ± 10 to 102 ± 9 mmHg at the end of the study (p<0.0001).
Systolic and diastolic blood pressure also decreased significantly during the follow-up (12 weeks) (p<0.001).
Losartan in monotherapy controlled blood pressure in more than 70% of transplanted patients from this study. Clinical and biochemical tolerance of losartan was excellent in all patients, with only slight increases in serum creatinine and potassium, without clinical relevance. In two cases, a mild normocytic anemia was reported, and in three other patients a mild impairment of preexisting anemia was also observed. No interactions with immunosuppressive therapy (cyclosporine and tacrolimus) were observed, and blood levels and doses of these drugs did not significantly change during the study period. From this study, losartan showed excellent efficacy and safety in the treatment of hypertension in renal transplant recipients, with good control of blood pressure, without interactions with immunosuppressive drugs and without major side effects (16).
Chronic allograft nephropathy and TGFß production
The principal cause of late graft loss after the first year of renal transplantation is chronic allograft nephropathy (6). Renal transplantation with chronic allograft nephropathy exhibits a gradual and progressive deterioration in renal function in association with proteinuria and arterial hypertension (17). Histology is characterized by arterial intimal and medial fibrosis and arteriolar insudative lesions, glomeruloesclerosis and interstitial fibrosis with tubular atrophy (18). Although chronic rejection has traditionally been seen as repeated low-grade immune responses directed against allogeneic tissue, recent evidence indicates that non-immunological factors may greatly contribute to its pathogenesis, especially arterial hypertension, lipid abnormalities, cyclosporine nephrotoxicity and advancing age in the graft donor (19). Recent evidence has also shown that TGF-ß1 is involved in the pathogenesis of chronic renal allograft dysfunction (20,21), and Amuchastegui et al. demonstrated that AngII receptor antagonists are clearly better than calcium channel blockers in the protection of the progression of chronic allograft nephropathy in an experimental model (22). TGF-ß1 plays a leading role in the development and progression of renal injury, inducing interstitial fibrosis, glomeruloesclerosis and arterial proliferation, and it leads to a glomerular hyperfiltration syndrome with arterial hypertension and proteinuria. Production of TGF-ß1 in these circumstances may be modulated by the intrarenal renin-angiotensin system (RAS), since angiotensin II (Ang II) induces TGF-ß1 production and secretion by the mesangial cells, and by a direct effect of cyclosporine A, which stimulates the synthesis and expression of TGF-ß1(Figure 1)
In a prospective study with fifteen renal transplant patients on chronic graft nephropathy we have demonstrated that the treatment with Losartan significantly decreased the plasma levels of TGF-ß1 (Figure 2) (23) The decrease in serum TGF-ß1 was greater than 50% with respect to the basal levels, and at the end of the study there was no significant difference with respect to the control group of transplanted patients with normal renal function on cyclosporine therapy. The reduction in TGF-ß1 was observed in all patients and was progressive throughout the study period. The reduction in TGF-ß1 observed in the present study is fairly consistent with the data from experimental animals -about 50%- although in most of these studies TGF-ß1 levels remained somewhat high. The mechanism by which losartan decreased serum levels of TGF-ß1 is through Ang II blockade. We demonstrated a significant correlation between the increase in AngII at 2 weeks and the decrease in TGF-ß1 at the end of the study period, suggesting that the AT1 receptor blockade plays a decisive role in the synthesis of TGF-ß1.
We have also observed a significant decrease in endothelin-1 serum levels during the treatment with Losartan, which could contribute to the control of blood pressure (23). Furthermore, the decrease in TGF-ß1 and endothelin could have an important and decisive protective role in the progression of chronic allograft nephropathy.
Proteinuria
Proteinuria represents one of the strongest risk factors for the progression of chronic renal failure and cardiovascular events and has been consistently associated with the development of chronic allograft nephropathy (24,25). Indeed proteinuria itself may contribute to the progression of renal disease. A large number of potential mechanisms have been ascribed to the relationship between proteinuria and progressive injury (26). The excessive filtered plasma proteins are, in part, reabsorbed by proximal tubule cells and, in that process, various small molecules and substances bound to the proteins are liberated. In particular, the removal of iron from transferrin may provide a catalyst for the synthesis of locally damaging reactive oxygen species. Small lipids bound to these filtered proteins and freed during protein reabsorption may also have inflammatory or chemotactic properties which would accentuate tubulointerstitial disease. Finally, the inspissation of filtered proteins due to water abstraction in the distal nephron may lead to cast formation and intrarenal obstruction. For all these reasons, reduction of proteinuria has been considered as a subrogate marker for renal protection. Although the reduction in proteinuria can be related to lowering blood pressure, it appears that drugs blocking the renin-angiotensin system have an effect that is beyond their antihypertensive effect (25,26). This has been clearly demonstrated with ACE inhibitors, and more recently with AngII receptor antagonists, although there are no references in the literature about this effect in the renal transplant population (26-28).
From the Spanish multicenter study of hypertension in renal transplant patients, we evaluated the effect of Losartan on the treatment of proteinuria in a subset of recipients (21 patients) with clinically relevant proteinuria (> 300 mg/d) (16). Losartan significantly reduced the proteinuria from 2.0 g/d to 0.8 g/d at the end of the study (Figure 3).
This reduction was initially observed at two weeks after the introduction of losartan, the maximum reduction being after 6 weeks of losartan therapy. In the study of chronic allograft nephropathy, five out of fifteen included patients had marked proteinuria (> 1 g/d), and in all of these five recipients a significant decrease in proteinuria was observed. In summary, losartan was highly effective in the reduction of proteinuria in renal transplant patients.
Summary
Renal transplantation represents the first option in the treatment of patients with end-stage renal failure, with a high improvement in the quality and expectancy of life with respect to the dialysis therapy in this population. Notwithstanding, in the last few years, an increase in cardiovascular mortality and morbidity has been reported in this population, hypertension being one of the most important and prominent factors in this negative action. Moreover, chronic allograft nephropathy still increased in recent years, despite a dramatic decrease in the incidence of acute graft rejection and the introduction of new immunosuppressive drugs. In this scenario, the activation of the intrarenal renin-angiotensin system could have a predominant role collaborating directly in the development of hypertension and renal injury. For these reasons, the use of AngII receptor antagonists in renal transplant recipients seems essential, with losartan demonstrating a great efficacy and safety in the treatment of hypertension, proteinuria and presenting promising results in the treatment and prevention of chronic allograft nephropathy through a decrease in the levels of TGF-ß1. These results in the treatment and prevention of chronic allograft nephropathy need to be confirmed in larger and longer experiments, and there seems to be a need for studies to prevent the development of graft nephropathy using Ang II receptor antagonists, especially in high risk populations.
References
-
1. Ponticelli C, Montagnino G, Aroldi A, Angelini C, Braga M, Tarantino A. Hypertension after renal transplantation. Am J Kidney Dis 1993;21 (5 Suppl 2):73-8.
2. Mailloux LU, Haley WE. Hypertension in the ESRD patient: pathophysiology, therapy, outcomes, and future directions. Am J Kidney Dis 1998;32:705-19.
3. Opelz G, Wujciak T, Ritz E, for the Collaborative Transplant Study. Association of chronic kidney graft failure with recipient blood pressure. Kideny Int 1998;53:217.222.
4. Aker S, Ivens K, Grabensee B, Heering. Cardiovascular risk factors and diseases after renal transplantation. Int Urol Nephrol 1998;30:777-88.
5. Koch M, Gradaus F, Schoebel FC, Leschke M, Grabensee. Relevance of conventional cardiovascular risk factors for the prediction of coronary artery disease in diabetic patients on renal replacement therapy. Nephrol Dial Transplant 1997;12:1187-91.
6. Kasiske BL. Clinical correlates to chronic renal allograft rejection. Kidney Int 1997; 52 (Suppl 63):S71-S74
7. Mihatsch MJ, Kyo M, Morozumi K, Yamaguchi Y, Nickeleit V, Ryffel. The side-effects of ciclosporine-A and tacrolimus. Clin Nephrol 1998;49:356-63.
8. Oriji GK, Keiser HR. Role of nitric oxide in cyclosporine A-induced hypertension. Hypertension 1998;32:849-55.
9. Ivic MA, Stefanovic V. Endothelins in hypertension and kidney diseases. Pathol Biol (Paris) 1998;46:723-30 .
10. Shihab FS, Bennet WM, Tanner AM, Andoh TF. Angiotensin II blockade decreases TGF-ß1 and matrix proteins in cyclosporine nephropathy. Kidney Int 1997;52: 660-673.
11. Khanna A, Kapur S, Sharma V, Li B, Suthanthiran M. In vivo hyperexpression of transforming growth factor ß1 in mice: stimulation by cyclosporine. Transplantation 1997;63:1037-1039.
12. Border WA, Noble NA. Interactions of transforming growth factor-ß and angiotensin II in renal fibrosis. Hypertension 1998;31:181-188.
13. Curtis JJ. Treatment of hypertension in renal allograft patients: Does drug selection make a difference ? Kidney Int 1997; 52 (Suppl 63):S75-S77.
14. Mourad G, Ribstein J, Mimram A. Converting-enzime inhibitor versus calcium antagonist in cyclosporine-treated renal transplants. Kidney Int 1993;43:419-23.
15. van der Schaaf MR, Hene RJ, Floor M, Blankestijn PJ, Koomans HA. Hypertension after renal transplantation. Calcium Channel or Converting Enzyme Blockade? Hypertension 1995;25:77-81.
16. Castillo D, Campistol JM, Guirado L, Capdevilla L, Martínez JG, Pereira P, Bravo J, Pérez R. Efficacy and safety of losartan in the treatment of hypertension in renal transplant recipients. Results of a multicenter study. Kidney Int 1998; 54 (Suppl.68):S135-S139.
17. Matas AJ, Burke JF Jr, DeVault GA Jr, Monaco A, Pirsch JD. Chronic rejection. J Am Soc Nephrol 1994;4(8 Suppl):S23-S29.
18. Solez K, Axelsen RA, Benediktsson H, Burdick JF, Cohen AH, Colvin RB, Croker BP, Droz D, Dunnill MS, Halloran PF, et al. International standardization of criteria for the histologic diagnosis of renal allograft rejection: the Banff working classification of kidney transplant pathology. Kidney Int 1993;44:411-422.
19. Matas AJ. Chronic rejection-definiton and correlates. Clin Transplant 1994; 8:162-167.
20. Border WA, Noble NA. Transforming growth factor ß in tissue fibrosis. N Engl J Med 331(19): 1286-1292,1994.
21. Shihab FS, Tanner AM, Shao Y, Weffer MI. Expression of TGF-beta 1 and matrix proteins is elevated in rats with chronic rejection. Kidney Int 50:1904-1913,1996.
22. Amuchastegui SC, Azzollini N, Mister M, Pezzotta A, Perico N, Remuzzi G. Chronic allograft nephropathy in the rat is improved by angiotensin II receptor blockade but not by calcium channel antagonism. J Am Soc Nephrol 1998;9:1948-55.
23. Campistol JM, Iñigo P, Jimenez W, Lario S, Clesca PH, Oppenheimer F, Rivera F. Angiotensin II receptor antagonist (losartan) decreases plasma levels of TGF-ß1 in transplant patients with chronic allograft nephropathy. Kidney Int (in press)
24. Paul LC, Sijpkens YW. Surrogate end points in chronic kidney graft rejection studies. Transplant Proc 1999;31:1293-4.
25. Remuzzi G, Bertani T. Pathophysiology of progressive nephropathies. N Engl J Med 1998;339:1448-56.
26. Thomas ME, Brunskill NJ, Harris KP, Bailey E, Pringle JH, Furness PN, Walls J. Proteinuria induces tubular cell turnover: A potential mechanism for tubular atrophy. Kidney Int 1999;55:890-8.
27. Maschio G, Alberti D, Locatelli F, Mann JF, Motolese M, Ponticelli C, Ritz E, Janin G, Zucchelli P. Angiotensin-converting enzyme inhibitors and kidney protection: the AIPRI trial. The ACE Inhibition in Progressive Renal Insufficiency (AIPRI) Study Group. J Cardiovasc Pharmacol 1999;33 (Suppl 1):S16-20.
28. Preston RA. Renoprotective effects of antihypertensive drugs. Am J Hypertens 1999;12(1 Pt 2):19S-32S.
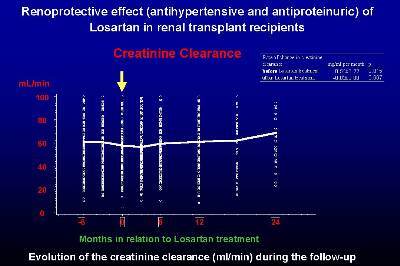 Figure 1
Figure 1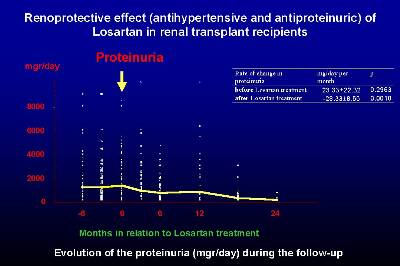 Figure 2
Figure 2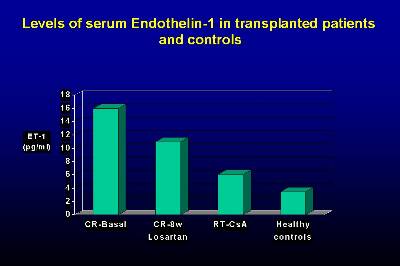 Figure 3
Figure 3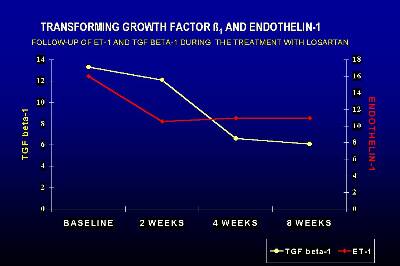 Figure 4
Figure 4