PANEL DE DISCUSION |
a solution that depends on integrative physiology
Mitchell L. Halperin MD, FRCP(C) and Kamel S. Kamel MD
Renal Division, St. Michael’s Hospital, University of Toronto, Toronto, Ontario, Canada
Email: mitchell.halperin@utoronto.ca
Abstract
In clinical medicine, information is provided in discreet bits. In contrast, viewing the overall picture requires an integration of information and concepts derived from a number of different areas. When in-depth comprehension is sought, the challenge is to examine data from a number of ‘compartmentalized’ areas, but think in a broad and integrative fashion. This general theme is illustrated using basic information concerning the major effects of aldosterone on the kidney. The integrative picture became clearer after the traditional interpretations of acid-base balance were challenged. At the clinical level, this global view can help us understand why a low intake of potassium might predispose to an expanded extracellular fluid volume and hypertension in patients whose blood pressure rises in response to an expansion of their vascular volume.
Key words: Acid-base, hypertension, integrative physiology, kidney stones, potassium, sodium
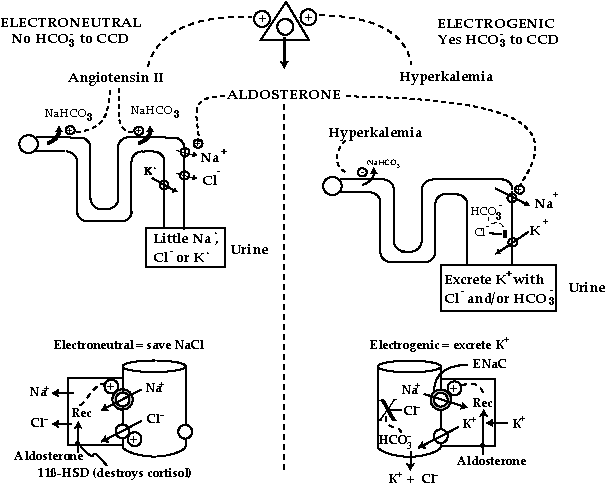
In clinical medicine, all the information provided by a patient must be examined carefully to reach a final diagnosis and plan for its therapy. A similar comprehensive strategy is needed when considering an altered pattern of physiology. Therefore our emphasis in this article will be on a broad-based view of a problem. We shall focus initially on the action of the hormone aldosterone with its unique signal-response system (Figure 1) . The first element in the system is a sensor to detect either a deficit of sodium chloride (NaCl) or a surplus of potassium (K+). A deficit of Na+ is sensed by cells of the juxtaglomerular apparatus (JGA) that release renin in response to a contracted ‘effective’ extracellular fluid (ECF) volume . The sensor is connected to the next component by means of angiotensin II (AII) which is delivered to the adrenal cortex. Once AII binds to adrenal cells, aldosterone is released into the blood. A second and largely independent sensor for aldosterone release from the adrenal cortex is a surplus of K+.
By placing receptors for hormones in some organs and not others, or using different downstream signal-transduction systems in individual cell types, a single hormone can have different actions in specific t target areas. In general, these receptors are highly specific for their hormone ligand. Nevertheless, the renal receptor for aldosterone is not specific for this hormone. Hence a second strategy is needed to ‘select’ specificity for aldosterone. Surrogate receptor agonists such as cortisol are prevented from reaching the aldosterone receptor. For example, cortisol is catabolized by a pair of enzymes called 11 ß-hydroxysteroid dehydrogenase (11-ßHSDH) which destroy cortisol before it can reach the aldosterone receptor in principal cells of the cortical collecting duct (CCD) (Figure 1).
There is only one major ultimate target. Once aldosterone binds to its receptor in principal cells of the CCD, a sodium ion (Na+) channel in their luminal membrane (ENaC) becomes activated (Figure 1) . Since aldosterone could have been released in response to two different sensors, two mutually exclusive renal responses are required. Hence if there is a contracted ‘effective’ circulating volume, the desired renal response is to initiate the retention of NaCl in the body. In contrast, if there is a surplus of K+ in the body, the desired response is to promote the excretion of K+.
The question we shall address is: how can aldosterone have a single overall function if it has two distinct stimuli for its release as well as two separate potential renal responses? For example, are we condemned to renal K+ wasting just because we need to retain NaCl or can we make aldosterone become a NaCl-retaining hormone without causing an unwanted excretion of K+ if there is both a deficit of NaCl and K+? The converse should also apply when we eat a meal rich in NaCl and K+. Therefore this question can be restated: "Is there a way to select the NaCl-retaining Vs. the kaliuretic actions of aldosterone? Will aldosterone itself be able to make this differentiation? If not, what other element should deliver this specific message?"
We have considerable insights as to what aldosterone does in molecular terms in principal cells of the CCD. Simply put, its major action is to activate ENaC . Nevertheless, an open ENaC is needed for both of the major actions of aldosterone (Figure 1). Hence control of ENaC cannot select for NaCl-retaining and not kaliuretic actions of aldosterone. A glance at the CCD in the bottom portion of Figure 1 reveals that control of chloride (Cl-) transport systems and/or K+ conductance across the luminal membrane of principal cells offers potential strategies for ‘selecting’ the ‘either/or’ needs of the body with respect to actions of aldosterone.
Clinical observations can also help us to understand how aldosterone can be either an NaCl-retaining or K+-secretory hormone. For example, in many K+-wasting states, bicarbonaturia is a common feature (distal RTA , vomiting , administration of carbonic anhydrase inhibitors, diurnal pattern of K+ excretion with its peak at the time of the alkaline tide ). This led to the speculation that luminal bicarbonate ions (HCO3-) and/or an alkaline luminal pH could regulate Cl- reabsorption or K+ conductance. This effect of HCO3- to ‘select’ either the NaCl-retaining or kaliuretic actions of aldosterone by the absence or presence of HCO3- in the lumen of the CCD is unique compared to other anions. For example, sulfate anions are not reabsorbed in the distal nephron. Nevertheless, they stimulate K+ secretion only when the luminal [Cl-] is very low . HCO3-, on the other hand, seems to stimulate the secretion of K+ even when abundant Cl- is present in the lumen of the CCD.
The aldosterone paradox:
The hypothesis we have put forward is that two physiologic stimuli for the release of aldosterone can deliver their ‘messages’ via a controlling delivery of HCO3- to the CCD (Figure 1). When there is a low effective circulating volume, the release of aldosterone is stimulated by AII. Aldosterone can function as an NaCl-retaining hormone because AII, its secretagogue, stimulates the reabsorption of HCO3- by the proximal and distal tubules . Hence, AII diminishes the kaliuretic effect of aldosterone by lowering distal delivery of HCO3-. In contrast, when aldosterone is needed to promote the excretion of K+, having hyperkalemia as its secretagogue is appropriate. In this case, not only will the release of aldosterone be stimulated, but also hyperkalemia will inhibit HCO3- reabsorption in the PCT . A high distal delivery of HCO3- will select the electrogenic reabsorption of Na+ in the CCD (Figure 1).
Central role for HCO3- in the luminal fluid of the CCD:
There are obvious consequences of utilizing HCO3- or an alkaline pH in the lumen of the CCD to select the kaliuretic and not the NaCl-retaining actions of aldosterone (Figure 1). For if HCO3- are to be an important signal in the CCD, there must be other ways to excrete an alkali load if one does not want to excrete K+ at that time. This in turn requires an appreciation of the integrative physiology of acid-base balance that emphasizes how the body deals with the alkali load in the diet.
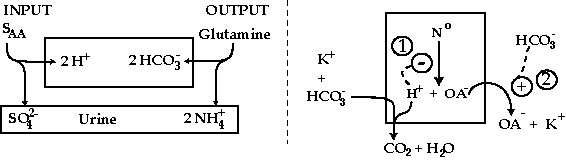
Unfortunately, the traditional view of acid-base balance is not a ‘balanced’ view. It focuses on H+ production and elimination (ammonium (NH4+) excretion) which implies that the diet produces a net acid load from metabolism of dietary constituents . This view is only a partial truth because it ignores the fate of the alkali load of the diet that can be quite large indeed if more fruits and vegetables are consumed . The traditional view is that the alkali load will be disposed of by the excretion of HCO3- because its maximum for tubular reabsorption is exceeded. Hence the renal handling of base is measured as HCO3- excretion in the customary definition of net acid excretion. Our interpretation differs from the traditional view with respect to dietary alkali (e.g., K+ salts of organic anions). A HCO3- load will be generated and removed by metabolic means when organic acids are formed and their conjugate base are made into end-products of metabolism (Figure 2). In more detail, alkali will stimulate the production of H+ (e.g., citric acid) and then augment the excretion of citrate anions in the urine along with the cation with which alkaline salts entered the body. When this system is viewed as a larger picture (Figure 2), bicarbonaturia is not a necessary consequence of consuming a reasonably sized alkali load because alkali prevents the reabsorption of citrate in the PCT , thereby obligating its renal excretion (Figure 2). Even when the diet produces a net H+ load, distal delivery of HCO3- is still possible if a kaliuretic response is required because the ability to raise the excretion of NH4+ is large in response to a large, chronic acid load .
The traditional concept of a tubular maximum and a renal threshold for renal HCO3- reabsorption must be re-evaluated because two procedures may be used to elevate the plasma [HCO3-]. The first is the addition of NaHCO3 and the second has HCO3- added to the body along with the loss of an equivalent amount of Cl- for electroneutrality (selective loss of HCl). Both processes could lead to similar elevations in the plasma [HCO3-], but renal HCO3- wasting only was appreciable with NaHCO3 . The major different between the two different HCO3- loads is the expanded ECF volume with suppression of renin and AII levels with NaHCO3 whereas the converse is likely with a HCO3-/Cl- anion exchange process .
One might also take a deductive approach to this problem of a HCO3- gain linked to a Cl- loss which is an anion exchange process . This type of anion exchange occurs during the gastric secretion and temporary retention of HCl in the stomach . In quantitative terms, human gastric secretion of HCl is close to 100 mmol per day. Very little of the 100 mmol of HCO3- that were added to the body appear in the daily urine. If bicarbonaturia was a prompt response to a temporary alkalemia without an expanded ECF volume, it could induce large renal losses of Na+ and/or K+. With little intake of Na+ salts, hypovolemia could be the result, whereas with little intake of K+, hypokalemia would develop. Moreover, later in time, the rate of NH4+ excretion would have to rise by an amount equal to the renal loss of HCO3-. Nath et al have suggested that chronic high rates of excretion of NH4+ could lead to renal medullary damage.
Acid-base physiologists are very familiar with the fact that the reabsorption of HCO3- in the PCT is augmented by hypokalemia . When this fact is considered in integrative terms (Figure 1), K+ deficiency will decrease distal delivery of HCO3-, thereby diminishing kaliuresis. With little distal delivery of HCO3-, there will be an enhanced reabsorption of NaCl when ENaC is open . As a result, the ECF volume will be higher and low-renin hypertension could ensue in patients whose blood pressure is more sensitive to blood volume than vasoconstrictors. Giving them a potassium chloride load leads to a negative balance for NaCl and a fall in their blood pressure .
Concluding remarks:
The NaCl-retaining and kaliuretic actions of aldosterone occur because of events in principal cells of the CCD with only one type of receptor and one major response element (activated ENaC). To segregate the two possible effects of aldosterone, a second form of control is needed downstream to opening ENaC. We hypothesized that this differential response could be mediated by varying the distal delivery of HCO3- and/or an alkaline lumen pH in the CCD. For this signal system to operate, one must have an alternate form of alkali excretion system such as the excretion of citrate anions. Moreover, one also needs a system to deliver HCO3- distally when there is a chronic acid load–this can occur if the excretion of NH4+ is augmented.
figure 1: selecting the nacl-retaining vs. the kaliuretic actions of aldosterone
The adrenal gland is depicted as a triangle at the top of the Figure. The secretagogues for aldosterone influence the reabsorption of HCO3- in upstream nephron segments to select the desired renal response in the nephron (middle section of the Figure). The events in the CCD are shown in the bottom section of the Figure. Aldosterone is delivered via the plasma, binds to mineralocorticoid receptors (REC) inside principal cells because 11-ßHSDH does not destroy this hormone. The hormone receptor complex leads to the insertion of active ENaC units (depicted by shaded enlarged ENaC).

figure 2
acid-base balance
For acid balance (left portion), H+ are produced when a neutral sulfur-containing amino acid (SAA) is converted to sulfuric acid. This H+ load is eliminated when the kidney converts the amino acid glutamine to HCO3- ions (retained) and NH4+ ions (excreted). Thus the urine contains NH4+ and sulfate anions (SO4+) in equivalent amounts. For base balance (right portion), when fruits or vegetables are ingested, K+ and HCO3+ ions are added to the body. The HCO3- ions lead to removal of H+ from the body, augmenting the production of organic acids such as citric acid (H+ + OA-) (site 1). HCO3- also cause the kidney to excrete anions like citrate (OA-) along with K+ (site 2). Thus the alkali load is eliminated in a form that minimized bicarbonaturia.
Address all correspondence to:
M.L. Halperin, MD, FRCP(C)
Division of Nephrology St. Michael's Hospital
38 Shuter Street Toronto, Ontario, Canada
Phone: (416) 864-5292 FAX: (416) 864-5943
![]()