PANEL DE DISCUSION |
IMMUNE MONITORING IN KIDNEY TRANSPLANTATION
Mariona Mestre and Jordi Bas
Servei d’Immunologia. Ciutat Sanitària Universitària de Bellvitge.
Barcelona. Catalonia.
In clinical transplantation (Tx), immunosuppressive strategies based on the depletion of T lymphocytes applied just before surgery and throughout the immediate post–Tx period promote the abrogation of allograft response in kidney transplant recipients as we have reported (1). This was demonstrated by the low incidence of acute rejection episodes (less than 20% in first three months) and the good clinical outcome (3-year actuarial graft survival higher than 80%) using an induction triple therapy with concomitant polyclonal (ALG, ATG) or monoclonal (OKT3) antilymphocyte antibodies, cyclosporin and steroids at low doses. These good clinical results prompted us to study the immune mechanisms leading to the allograft acceptance in kidney clinical transplantation. Several lines of evidence suggest that the long-term maintenance of a transplant may be associated to a modified immunoregulatory state that involve different immune mechanisms such as clonal deletion/anergy of alloreactive cells, suppressor T cell expansion or changes in the pattern of cytokine synthesis. In the present contribution we summarise our most relevant results from longitudinal studies obtained in the investigation of the immune response of kidney allograft recipients treated with antilymphocyte antibodies. We believe that to obtain reliable data on allograft response in clinical transplantation the design of the study is critical. Therefore, we prefer to perform longitudinal studies because the value of some reported findings in cross-sectional studies is hampered by the inter-individual variability and the fact that the changes may not be permanent, misleading the real course of the immunologic variations.
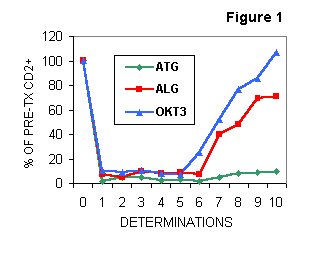
Besides early work performed by fluorescence microscopy (
2), ever since 1989 we have been monitoring PB lymphocyte subsets by flow cytometry. Sequential studies (Figure 1) were carried out within one month post-Tx, to assess 1) the efficacy of the therapy and 2) the selectivity of lymphocyte depletion and the behaviour of immunoregulatory cell subsets induced by different protocols. ATG-treated patients achieved the most intense and persistent depletion in each lymphocyte subset studied. In contrast, both ALG and OKT3 treated patients showed a depletion in all subsets during the triple therapy and recovered shortly after antibody cessation. In relation to pre-Tx, we observed that CD4 subset recovered earlier than CD8 subset (especially in OKT3 treated patients) and the recovery for CD4+CD45RA+ naive cells was faster than for CD4+CD45RO+ memory cells (3,4).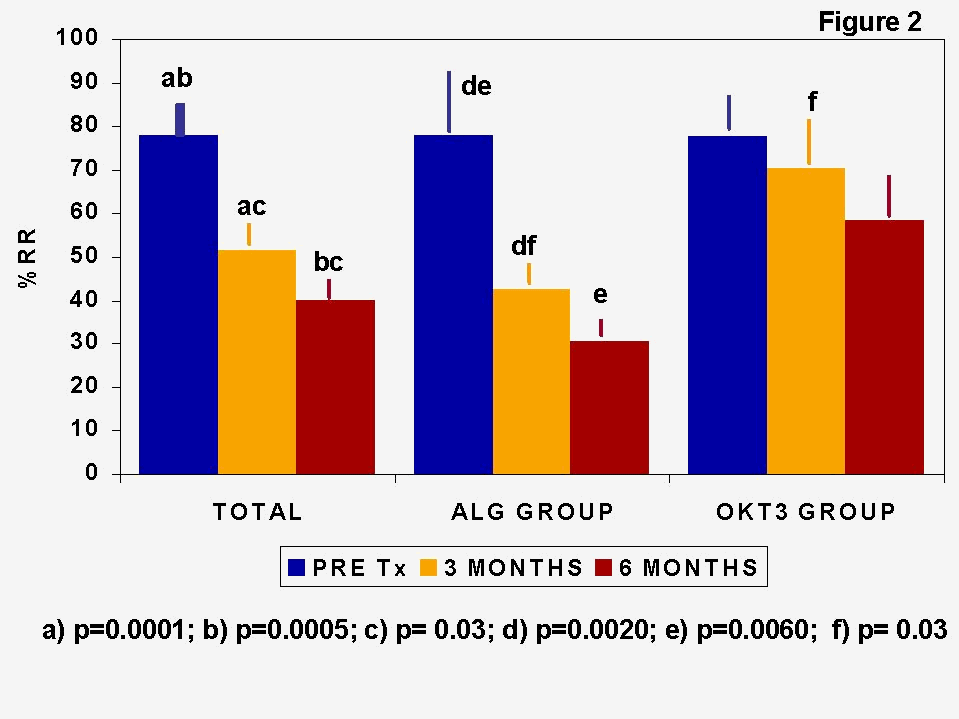
Immune monitoring studies consisting of mixed lymphocyte cultures (MLC) have shown that concomitant triple therapy induce a state of low donor-specific alloreactivity, as expressed in % of relative response (%RR), that was more marked in ALG-treated than in OKT3-treated recipients (Figure 2). MLC and immunoregulatory T cell subset quantification showed adaptive changes in the immune status of recipients which could promote allograft acceptance (
5,6). In order to clarify the phenomena underlying the changes in MLC responsiveness, MLC assays were performed pre-Tx and at 3 and 6 months post-Tx with PBL from ten allograft recipients against donor cryopreserved cells. At several time points (0, 3, 5 and 7 days) of culture we assessed the composition of lymphocyte subsets, the expression of activation markers and the production of cytokines (IL-2 and IFN-g ). The percentage of CD4+, CD4+CD45RO+, and CD8+ cells did not change along the MLC but slight increases in CD4+CD45RA+ cells were observed both in pre- and post-Tx cultures. It is noteworthy that, in pre-Tx cultures, CD8+CD45RA+ cells tended to decrease during MLC and that the inverse situation was observed in post-Tx cultures. The analysis of activation markers showed the existence of changes involving mainly HLA-DR expression by CD8+ lymphocytes. Pre-Tx cultures increased in both, percentage and level of expression, measured by the mean channel fluorescence, at MLC day 7. In post-Tx cultures, there were more CD8+HLA-DR+ cells at day 0 than observed in pre-Tx cultures but this percentage did not increase during MLC, although the intensity of expression did. It must be pointed out here that after Tx there was a lower level of expression of HLA-DR on both CD4 and CD8 T cells. This was probably due to the effect of CsA, as we observed in MLC cultures of healthy subjects in presence of CsA.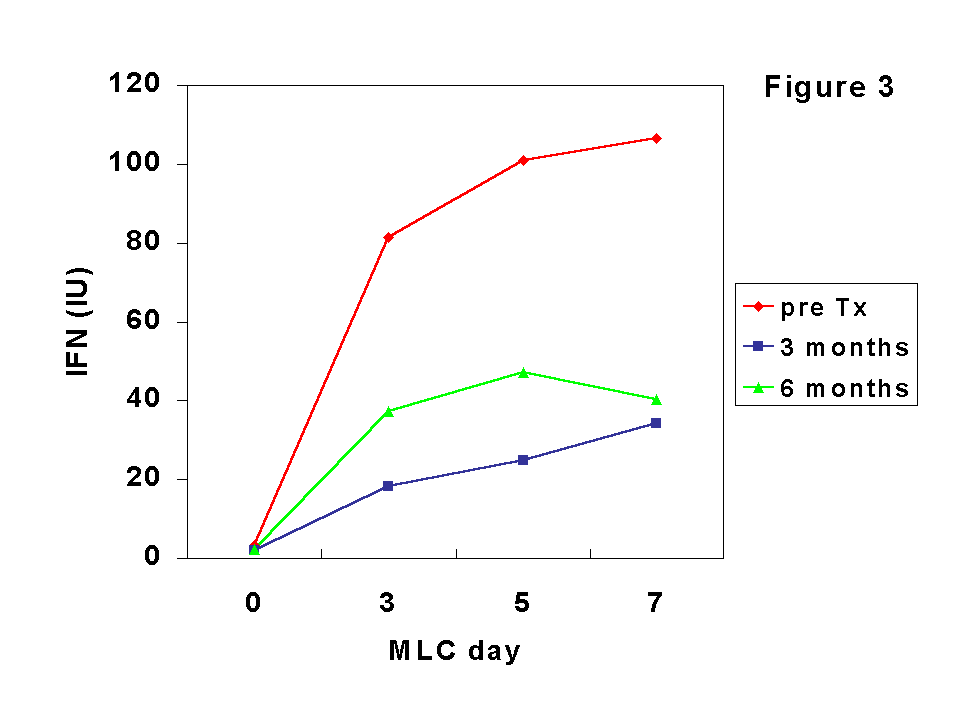
The effect of CsA may be mediated by the inhibition of IFN-
g since the production of this cytokine in MLC was clearly (although not totally) inhibited after Tx (Figure 3). In MLC, the increase in CD25+ cells occurred at a lower extent that the rise in HLA-DR expression, probably because allogeneic cells were poor inducers of IL-2 synthesis, as we observed in MLC from MHC-incompatible normal subjects. In the group of transplant recipients we found a relative increase of CD8+CD25+ cells along the culture that was more pronounced than those of CD4+CD25+ at both pre- and post-Tx. Furthermore, the increase of CD45RA+CD25+ cells was more pronounced at post-Tx culture while the inverse occurred with CD45RO+CD25+ cells, being in agreement with the expansion of CD8+CD45RA+ T cells observed in peripheral blood in our patients after Tx (7).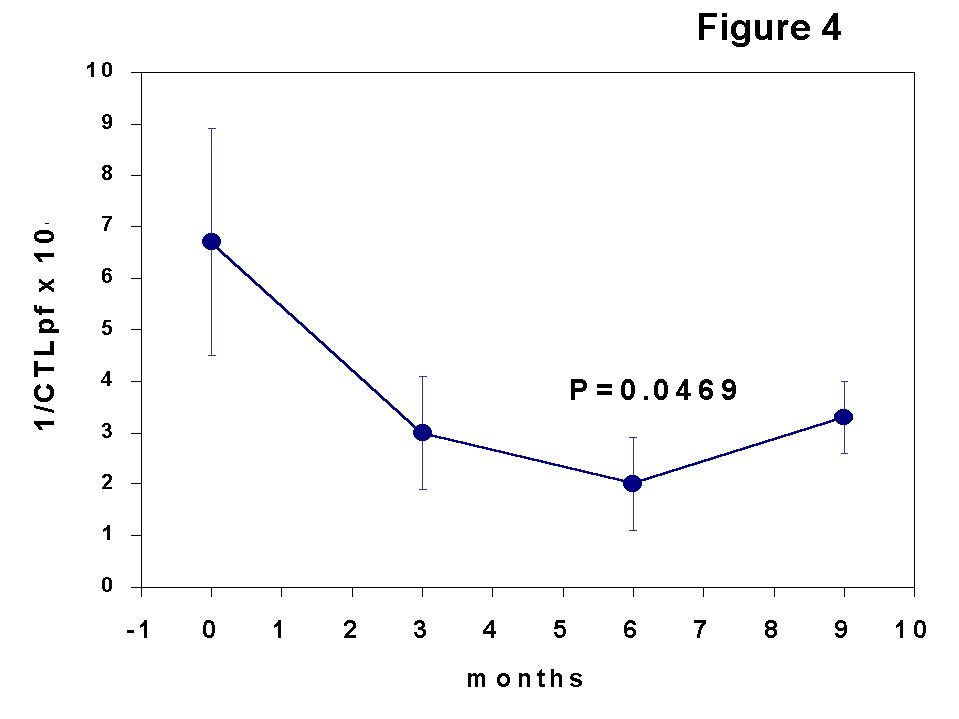
Another insight to investigate immune mechanisms involved in graft acceptance is the sequential determination of the frequency of cytotoxic T lymphocyte precursors (CTLpf) against donor alloantigens by limiting dilution analysis (LDA). This technique is useful to assess, at a clonal level, the adaptive changes occurring along the post-Tx period. We designed a sequential study consisting of the assessment of cytotoxic T lymphocyte frequencies (CTLpf) determined by limiting dilution analysis (LDA) and the PB lymphocyte subset quantification throughout the first year post-Tx in a group of kidney transplanted patients receiving concomitant triple therapy with antilymphocyte antibodies (
7).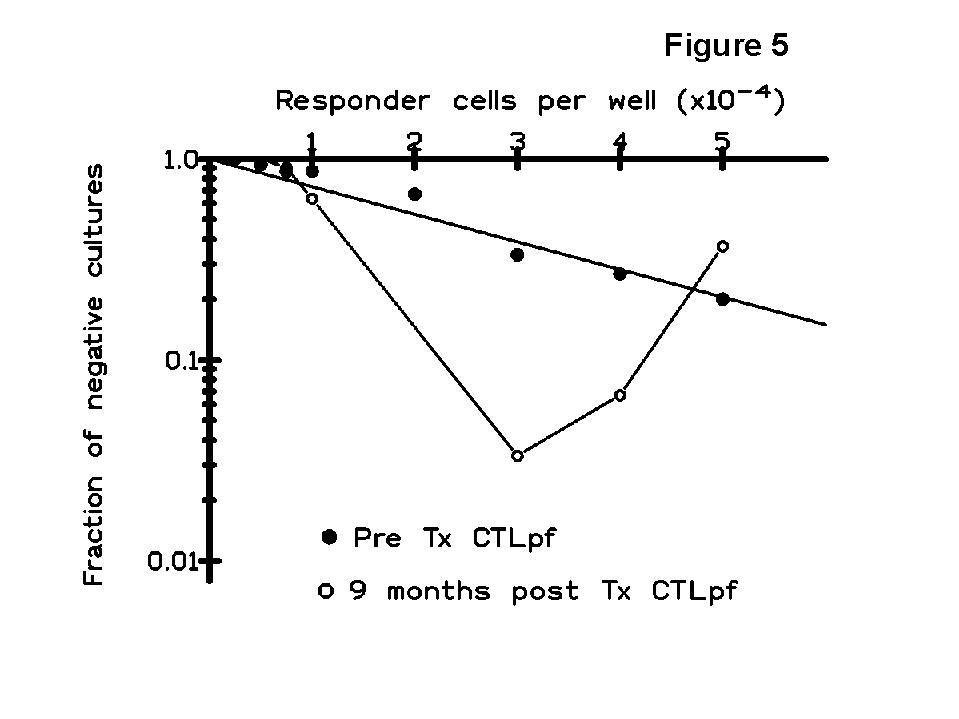
The kinetics of CTLpf showed a transient state of clonal functional inactivation (anergy) in the early post-Tx period with respect to pre-Tx values (Figure 4), and the changes in the results of T cell monitoring consisted of an expansion of a CD8 subset associated with suppressor function (CD8+CD45RA+ cells) and a shift in immunoregulatory CD4 subsets (memory cell decrease and sparing of naive cells). Since there was not a true and permanent deletion of T clones against the donor HLA antigens, other immune mechanisms such as the generation of suppressor cells may account for the maintenance of allograft acceptance. Indeed, the multi-hit curves (up-right inflexion) obtained in some patients of our study (Figure 5), detected in donor-specific but not in third-party LDAs, suggest the generation of suppressor cells as indicated by some authors (
8,9).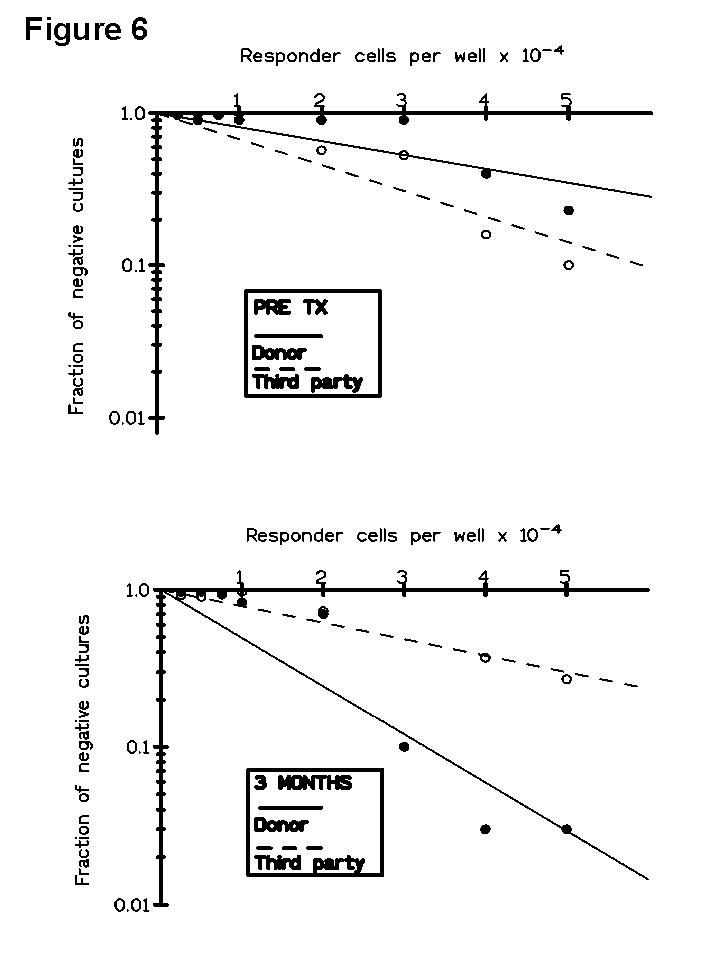
In a further LDA study (
10) we observed that although pre-Tx CTLpf estimation had no prognostic value, the post-Tx results obtained in patients with impaired renal function were related to clinical events. In Figure 6 is represented a LDA curve against donor and third-party obtained in a patient who lost her graft after rejection. In this way, three acute and one chronic rejection episodes were associated with a marked increase in anti-donor CTLpf, returning to pre-Tx values by 9 months post-Tx. It is noteworthy that all rejecting patients diminished anti-donor CTLpf after rejection, suggesting a mechanism of feed-back regulation of the alloresponse.It is known that the functionality of the immune system of renal transplant recipients is strongly conditioned by both the immunosuppressive therapy and the presence of the graft. Both factors may influence the generation of new T cells after the depletion caused by antilymphocyte antibody treatment and the cellular interactions during post thymic differentiation in the periphery. However, the adaptive mechanisms developed after recovery and their relevance to the outcome of the graft are still poorly understood. Accordingly, the phenotypic monitoring of peripheral blood (PB) lymphocyte subsets has demonstrated to be of great value in the identification of immunological changes occurring after transplantation. In order to explore whether or not patterns of changes in the distribution of PB lymphoid subsets and the expression of activation markers could be associated with the long-term clinical outcome of the grafts we performed a longitudinal study in kidney allograft recipients receiving triple therapy induction with antilymphocyte antibodies (ALG, OKT3) (
11). After severe depletion of lymphocytes in the first days post Tx, the kinetics and the pattern of the recovery of some T cell subsets in early post Tx were strongly related with the long-term graft function. Some post-Tx adaptive changes occurred during the first weeks, others within the first year, and other ones after 3 years. In this study a shift in the CD4/CD8 ratio occurred immediately after Tx and it was maintained during the follow-up. This change in the ratio was due to a huge increase in CD8+ lymphocytes. Thus, the effect of these changes was the relative reduction of the percentage of CD4+ T cell subsets. This relative decrease in the CD4+ compartment involved the CD4+ "memory" cells, expressing either CD45RO or CD29 molecules, whereas the percentage of CD4+CD45RA+ "naive" cells remained unchanged. We found a decrease of PB CD4+CD62L+ cells that is likely related to both, the decrease of CD45RO+ cells and the increased expression of activation-associated markers, which could downregulate this molecule in CD45RA+ cells.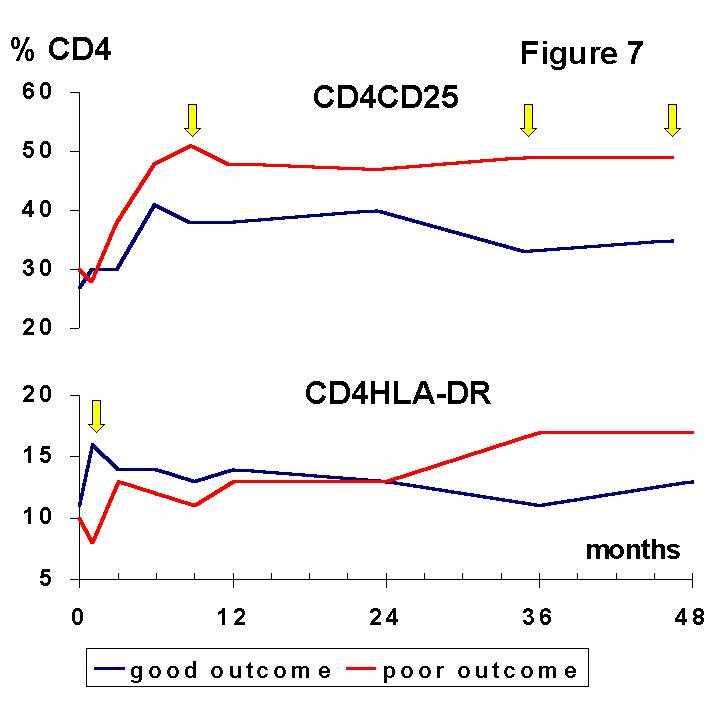
A good, stable renal function was related to the maintenance of low levels of CD4+ T cell subsets such as CD4+CD29+ and CD4+CD62L+ after the initial depletion of lymphocytes. In contrast, a worse outcome was associated with a rapid recovery of these subsets after the antilymphocyte antibody therapy. On the other hand, the recovery and consecutive expansion of CD8+ lymphocytes was faster and more important in patients with a good graft outcome. The expansion of the CD8+ compartment was mainly due to the steady increase in CD8+CD45RA+ "naive" cells. The CD8+CD45RO+ subset, mainly composed by cytotoxic effector cells, is predominating in rejected grafts (
12) whereas the CD8+CD45RA+, a putative suppressor-effector subset, is expanded after Tx (7,13) especially in patients with mixed lymphocyte culture hyporesponsiveness (5). Recently, CD8+ suppressor cell lines capable to prevent upregulation of costimulation B7 molecules on antigen presenting cells have been generated in vitro (14).The situation delineated by the balance of lymphocyte subsets may favour immune suppression and long-term graft acceptance. However, activation markers (Figures 7, 8) were increased in both, CD4+ and CD8+ T cells.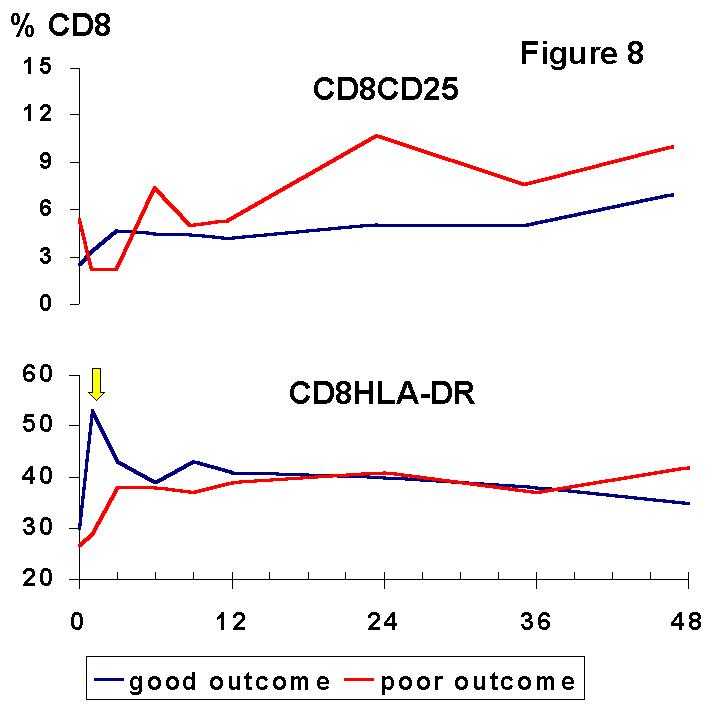
The most remarkable finding is the distinct meaning of the cellular activation observed during induction and maintenance phases of immunosuppression, suggesting that they reflect two unrelated phenomena. The absolute and relative increase of HLA-DR on CD4+ and CD8+ T cells during the first month post-Tx was associated with a good, stable renal function. We suggest that this early finding may be an expression of active mechanisms of immunoregulation which were not triggered in the patients with a poor graft outcome. The observed expression of HLA-DR by PB lymphocytes may be one feature of this immune response that may allow further immunomodulatory signals involved in graft acceptance. In this way, it is noteworthy that the expression of class II molecules can downregulate T cell response and induce tolerance to other alloreactive T cells. Furthermore, HLA class II signalling can mediate programmed cell death (
15), which may account for clonal deletion of alloreactive clones during early post-Tx, as we have observed in well functioning kidney recipients (7,10).In contrast, the persistence of high relative levels of activated CD4+ and CD8+ T cells from 3 years after Tx was associated with a poor renal function. This finding strongly suggests that transplantation can induce sustained activation of certain immune mechanisms in spite of the maintenance therapy and that the monitoring of activated subsets may be useful to identificate a group of patients at risk of chronic renal damage.
We conclude that immune monitoring in kidney transplanted patients provide detailed information of both in vitro and in vivo adaptive changes occurring post-Tx. The study of donor-specific alloresponse in mixed lymphocyte culture showed the acquisition of a state of donor-specific hyporesponsiveness in most patients that can be paralleled by changes in cytokine synthesis and variations of phenotype and activation markers in T lymphocytes. On the other hand, it is noteworthy tat the sequential assessment of peripheral blood T lymphocyte subsets and frequencies of cytotoxic precursors has shown to be associated to clinical events. Finally, to explain the peak of activation markers on T lymphocytes, especially HLA-DR expression at first month post-Tx in patients with good graft outcome, further studies are currently in progress in our laboratory.
REFERENCES
1
. Am J Kidney Dis 20:603-610, 1992.2
. Transplant Proc 20 (5) suppl 6: 52-55, 1988.3
. Transplant Proc 24 (1): 73-75, 1992.4
. Transplant Proc 31: 2254-2255, 1999.5
. Transplant Proc 24 (1): 76-77, 1992.6
. Allergol Immunopathol 21 (4): 136-140, 1993.7
. Transplant Proc. 27(4): 2371-2373, 1995.8
. J Immunol 144: 3748-3755, 1990.9
. Immunol Today 21(1): 15-18, 2000.10
. Clin Exp Immunol 104: 108-114, 1996.11
. Cytometry (Comm Clin Cytometry) 34:103-112, 1998.12
. Clin Exp Immunol 81: 225-231, 1990.13
. Transplantation 47: 465-471, 1989.14
. Int Immunol 10: 775-783, 1998.15
. Cell Immunol 172: 149-157, 1996.