Transplantation and short daily hemodialysis
J. TRAEGER*, R. GALLAND*, E. DELAWARI*, W. ARKOUCHE*
*AURAL (Association pour l’utilisation du rein artificiel) – Lyon –
10 Impasse Lindbergh – 69003 LYON
.
Introduction
The weekly frequency was once per week in the early days (1959-1960), then was 2 and 3 times weekly with long duration (12,10, 8 hours) using kiil dialyser (Scribner) until Cambi showed that 4 hours weekly was giving good clinical results. This strategy is known to be unphysiological. This is because every 2 days the humoral change variations are important.
For several years the unphysiology nature of the classical hemodialysis strategy has been stressed (1). Some daily hemodialysis assays have previously been done by Snyder (2) and Bonomini (3). Man in France was trying the daily strategy in children (4). Buoncristiani (5,6) has been working on this subject for 15 years, and has reported the excellent results which can be obtained. Kooistra in Holland (7), Ting in the USA (8), Uldall and Pierratos in Canada (9) have published interesting results.
We will report here the bio-clinical results obtained in our group using the 2 hours per day daily hemodialysis (DHD) strategy for more than 3 years.
Clinical results :
Ten patients previously treated with the standard strategy (SHD), 3 times, 4 to 5 hours per week, were transferred to a DHD strategy of 2 or 2.5 hours, 6 times per week. Weekly total dialysis duration remained the same. 4 patients are treated at home, 6 are treated in self
care unit. Mean age is 44.9 ±
13.8 years (21 –66). They have been hemodialyzed for 10.1 ±
6 – 7 years (1,19). And all are anuric.
The same hemodialysis modalities were used for both the 2 SHD and DHD periods, including the same machine, same dialyzer, identical blood flow, etc…. Blood access was the usual arteriovenous fistula using bipuncture.
Thus we could compare, in the same patient, the biological and clinical results obtained using the two hemodialysis strategies.
a) Blood pressure : 5 patients were hypertensive during the SHD period and were treated with antihypertensive medications. During the DHD period, blood pressure became normal and antihypertensive medications were stopped. Systolic, diastolic, and mean blood pressure decreased in all patients. (fig.1).
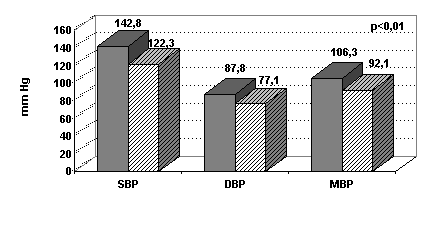
Figure 1 : Systolic blood pressure (SBP), diastolic (DBP) and mean (MBP)
on SHD and DHD
b) Left ventricular hypertrophy (LVH). Echocardiography before the DHD period showed LVH ; the LVMI > 134 g/m² in 8 patients. The mean value for the 10 patients was 190 g/m². The left ventricular diastolic diameter (LVDD) was more than 3.1 cm/m² in 7 patients. These are the usual results which are reported in the litterature (11).
During the DHD period, echocardiography performed every 6 months, showed a LVDD reduction and after one year only 2 patients had an abnormal LVDD. LVMI decreased in all patients after one year, from 172 to 131 g/m² (fig.2).
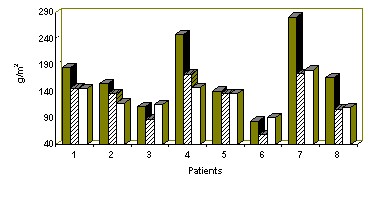
Figure 2 : LVMI in 8 patients who had more than one year with DHD. SHD at 6 months DHD at one year DHD
c) Nutritional status : Dietary inquiries were done every 3 months, showed 24 % increase in protein intake and 13 % increase in caloric intake. Albumin, préalbumin, transferrine and total cholesterol level are increased (table I). Dry weight also increased. (fig. 3).
| |
SHD |
DHD |
P |
|
Proteins intake (g /kg /day) |
1,32 ±
0,25 |
1,64 ±
0,49 |
0,031 |
|
Calories intake (Kcal/ kg/day) |
37 ±
9,93 |
42 ±
4,29 |
0,04 |
|
Albumin (g/l). |
38,5 ±
3,44 |
41,3 ±
3,03 |
0,0165 |
|
Pre albumin (g/l). |
0,33 ±
0,05 |
0,41 ±
0,10 |
0,0097 |
|
Transferrin (g/l). |
1,87 ±
0,23 |
2,26 ±
0,39 |
0,0174 |
|
Total Cholesterol (g/l). |
1,61 ±
0,30 |
1,79 ±
0,24 |
0,0153 |
Table 1: nutritional laboratory data.
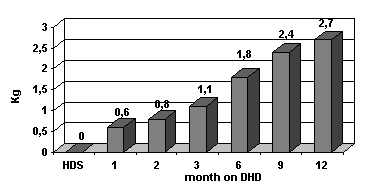
Figure 3 : Dry weight gain on DHD.
Corporeal mass index increases from 20.31 to 21.23 with higher lean and fat body mass.
nPCR is increased from 1.04 to 1.36 kg/day.
d) Anemia : During the SHD period, Hb level was 120 g/l with 7 patients being treated with EPO, mean dose 4000 u/week. During the DHD period, mean Hb level was 122 g/, EPO was stopped in 3 patients, and mean EPO dosage is now 1000 u/week.
e) Medication : During the DHD period, antihypertensive medications were reduced. We could reduce the dose of kayexalate by 66 % and the dose of phosphorus binders were reduced by 75 %.
f) Hemodialysis session tolerance : the overall tolerance is excellent. AVF puncture remained easy and the AVF blood flow remain inchanged (1634 ±
820 ml/mn SHD vs 1863 ±
628 ml/mn DHD).
No hypotension, or headache were observed despite an hourly higher ultrafiltration (with DHD of 0.79 kg/h vs 0.64 kg/h SHD).
g) Quality of life improved with DHD : there was no post-dialysis asthenia, less dietetic prescription, and less medication. Furthermore, the ultra short 2 hours sessions do not interfere with professional and social life.
Why these biological and clinical ameliorations ?
Two factors are involved : higher dialysis session frequency and higher hemodialysis dose.
A- Higher frequency reduces the hydrosodic interdialytic inflation. Ultrafiltration sessions are well tolerated and ideal dry weight was easily obtained (Table 2).
| |
SHD |
DHD |
|
D weight / session (kg) |
2,96 (1,94 - 4,30) |
1,8 (1,2 - 3,5) |
|
D weight / week (kg) |
8,9 (5,81 - 12,9) |
10,6 (7,2 - 19,2) |
|
UF / hour (kg / h) |
0,64 (0,49 - 1,08) |
0,79 (0,6 - 1,4) |
Table 2 : D weight and UF / hour
a) This allows better BP control.
b) The LVMI normalization appears to be due mainly to the reduction of interdialytic hydrosodic inflation, once the BP control has been obtained.
Anemia was not a factor in our patients since it was absent during the SHD period.
c) Better nutritional state is explained by disappearance of anorexia. This may be in relation with reduced medication and dietetic rules, more physical activity, and lower blood urea concentration.
d) Excellent dialysis session tolerance is probably due to the short 2 hours sessions, the lower interdialytic weight gain, and the lower blood/dialysate concentration gradients, which avoids disequilibrium syndrome.
B- Increase dialysis dose in DHD vs SHD. With the same weekly duration increased KT/V, lower TAC and lower TAD are part of the explanation for better clinical results.
a) Urea retention is less with DHD vs SHD. Urea concentration before the hemodialysis session is 23 % less, despite the only 13 % less urea concentration after the session. TAC urea is diminished by 17.12 % at 14.70 mmol/l (table 3), despite the increased protein intake.
b) TAD urea is reduced from 4.34 to 2.5 mmol/l, a 41 % lowering (table 3). This reduced TAD is an index of the more physiological aspect of DHD. Urea TAD is dependant only on hemodialysis frequency.
| |
SHD |
DHD |
P |
|
Urea pre dialysis (mmol/l) |
25,81 ±
4,83 |
19,84 ±
4,02 |
0,00079 |
|
Urea post dialysis (mmol/l) |
8,43 ±
1,65 |
9,55 ±
2,03 |
0,0887 |
|
Urea TAC (mmol/l) |
17,12 ±
3,08 |
14,70 ±
2,99 |
0,0185 |
|
Urea TAD (mmol/l) |
4,34 ±
0,94 |
2,57 ±
0,54 |
< 0,00001 |
|
URR ( % ) |
67 ±
4 |
52 ±
3 |
< 0,00001 |
Table 3 : Urea retention and urea TAD
c) KT/V was measured once a month with a urea monitor (UM 1000 Baxter) and dialysate sampler (Quantispal Hospal). More precisely, eKRn (Casino) (12,13) and standard KT/V (Gotch) (14) were calculated in order to have a better appraisal of dialysis dose.
KT/V during a 2 hour session vs 4 hour session diminished from 1.36 to 0.75. But, the weekly KT/V increased from 4.09 (SHD) to 4.51 (DHD). This was due to higher efficiency during the first 2 hours dialysis session.
eKRn and Sdt (KT/V) increased from 12.79 to 20.75 ml/mn (eKRn) and from 2.14 to 3.93 Sdt (KT/V) respectively. This last value expressed as the percentage of normal kidney Std (KT/V) is twice as great in DHD vs SHD, and reached 30 % of the normal kidney function (Table 4).
|
SHD |
DHD |
P |
|
eKt/V / session |
1,36 ±
0,26 |
0,75 ±
0,07 |
< 0,00001 |
|
eKt/V / week |
4,09 ±
0,77 |
4,51 ±
0,44 |
<0,00001 |
|
Sdt (Kt/V) |
2,14 ±
0,22 |
3,93 ±
0,59 |
<0,00001 |
|
eKRn (ml/min) |
12,79 ±
1,50 |
20,75 ±
3,54 |
<0,00001 |
|
% ref Sdt (Kt/V) |
16,51 ±
1,66 |
30,35 ±
4,58 |
<0,00001 |
Table 4 : Dialysis dose
Daily hemodialysis : an adequate hemodialysis strategy :
Until now, the main factors of dialysis adequacy were considered to be the dialysis dose as measured by the KT/V index (15,16,17). But, this index gives no indication or the type of dose delivery. For example, it could be done in one long session per week. But, this would be highly unphysiological.
A search for new markers of the physiological characteristics of hemodialysis was done by Valek and Lopot (18). In fig. 5 is shown a representation of different dialysis strategies on a TAD/TAC diagram. DHD appears to be nearest the zero origin which characterizes a normal individual with both functioning kidneys.
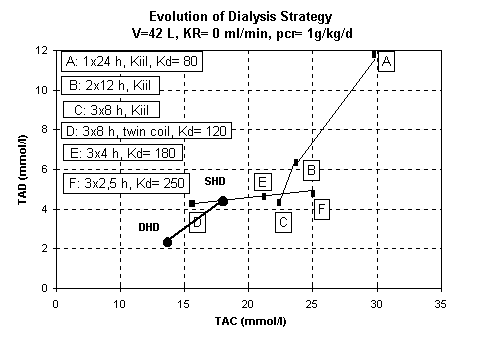
Figure 4 : TAC, TAD and hemodialysis strategy (Lopot) and our resultats
Moreover, increasing the frequency decreases the interdialytic hydrosaline inflation which is probably the main factor of dialysis intolerance, and part of the unphysiological aspect of SHD.
Is short daily hemodialysis strategy possible in the long term ?
a) Needling : In our experience, daily punctures of the AVF with two needles was done with no additional complications. Among 10 patients, 4090 punctures were done. There were only 9 incidents. One AVF had to be revised.
b) Patients are satisfied with this new strategy. None of them wants to return to SHD. Session tolerance is excellent, and there is no asthenia post-dialysis. These are the main arguments.
c) Short daily hemodialysis is best performed at home. But, it is also possible to do in a self care unit (20).
Should short daily hemodialysis be recommended as pretransplant preparation ?
A) 20 % SHD patients have some complications : these include hypertension, left ventricular hypertrophy and undernutrition. These complications are difficult to control even with well established SHD (KT/V > 1.3). As shown before, these complications resolve using DHD stragey. This is mainly because the interdialytic weight gain is low and because dry weight is obtained easily. Blood pressure approaches normal after one month. Two months are necessary to resolve or considerably ameliorate LVH. Anorexia disappears after some days with DHD strategy, and undernutrition resolved in 2 months with a rising nPCR.
B) Cardiovascular accidents in the post transplant period (mid or long term) are the main cause of death (more than 50 %). Coronary artery disease, hypertension, and left ventricular hypertrophy are the identified factors (21,22,23).
It is more difficult to assess the role of these factors in the immediate post transplant period and no detailed statistics are available (24).
It is also difficult to assess statistically the exact role of under nutrition in the immediate, mid-term or long-term post transplant period. Common sense tells us that good nutritional status would improve transplanted patients outcome, especially in cases where during infectious or surgical complications are present after transplantation.
C) Expected advantage of using DHD strategy in the pretransplant period would be :
1- Reintegration on the waiting list of patients whose transplant indications are decreased due to undernutrition, hypertension, cardiac insufficiency with LVH.
2- Diminution of post-transplant cardiovascular complications in normotensive patients with normalized cardiac volume obtained with DHD strategy.
3- Better resistance to surgical complications, in patients with a good nutritional state.
D) Unexpected undesirable effects should be carefully looked at (25,26,27)
- more delayed or primary non graft function due to the normal hydration of patients being on ideal dry weight. Prevention could be easily done by over hydration immediately prior to transplantation (28).
- good nutritional state could eventually lead to more immunocompetent patients with
a higher incidence of acute rejection, as happens in patients on peritoneal dialysis (25).
Conclusions :
At the present time, short DHD is the most adequate hemodialysis strategy. DHD leads to resolution of cardiovascular complications such as hypertension and left ventricular hypertrophy. With DHD, undernutrition is rapidly overcome with a rising nPCR and dry weight.
Short DHD appears to be a useful strategy to improve the clinical state of pretransplant patients. DHD would allow more patients to be put on the waiting list. DHD would probably reduce the cardiovascular complications in the post transplant period, and make the patients more resistant in the case of infectious or other surgical complications.
ACKNOWLEDGMENTS
The authors thank Mr. R. Hadden for the correction of the manuscript.
REFERENCES
1. Kjellstrand CM, Evans RL, Petersen RJ, Shideman JR, Von Hartitzsch B, Buselmeier TJ.
The unphysiology of dialysis : A major cause dialysis side effects ? Kidney Int. 1975; 7 (2) : S30-S34.
2. Snyder D, Louis BM, Gorfien P, Mordujovich J. Clinical experience with long-term brief, daily haemodialysis. Proc. Eur. Dial. Transplant. Assoc. 1975; 11: 128-135.
3. Bonomini V, Mioli V, Albertazzi A, Scolari P. Daily dialysis program : Indications and results. Proc. Eur. Dial. Transplant. Assoc. 1972; 9: 44-52.
4. Naret C, Yanai M, Delons S, Man NK. Frequent and Short Duration Dialysis with Reference to Urea, Creatinine, and Phosphate Generation Rate, Kt/V-Urea, and PCR. ICAOT: Renal Therapy. 1992; 335-339.
5. Buoncristiani U, Quintaliani G, Cozzari M, Giombini L, Rugaiolo M. Daily dialysis : Long-term clinical metabolic results. Kidney Int. 1988; 33(suppl; 24): 137-140.
6. Buoncristiani U, Caizon G, Giombili L, et al. Dramatic improvement in clincial metabolic parameters and quality of life with daily dialysis. Int. J. Artif. Organs. 1989; 12: (suppl.24): 133-136.
7. Kooistra MP, Vos J, Koomans HA, Vos PF. Daily home haemodialysis in the Netherland : effects on metabolic control, haemodynamics and quality of life. Nephrol. Dial. Transplant. 1998; 13: 2853-2860.
8. Ting G, Freitas T, Saum N, Carrie B, Kjellstrans C. Early metabolic, haematological, clinical and life quality changes with daily hemodialysis. Peritoneal Dialysis Int. 1998; 18 (suppl.1): 578.
9. Uldall PR. Will daily hemodialysis be an important future therapy for end stage renal disease. Semin. Dial. 1995; 8: 268-270.
10. Traeger J, Galland R, Delawari E, Arkouche W. Daily versus standard haemodialysis : One year experience. Artif. Organs. 1998; 22(7): 558-563.
11. London G. Heterogeneity of left ventricular hypertrophy – does it have clinical implication ? Nephrol. Dial. and Transplant. 1998; 13: 17-19.
12. Casino FG, Lopez T. The equivalent renal urea clearance : A new parameter to assess dialysis dose. Nephrol. Dial. Transplant. 1996; 11: 1574-1581.
13. Depner TA. Benefits of more frequent diaysis : lower TAC at the same Kt/v. Nephrol. Dial. Transplant. 1998; 13(6): 20-24.
14. Gotch F. The current place of urea Kinetic Modelling with respect to different dialysis modalities. Nephrol. Dial. Transplant. 1998; 13(6): 10-14.
15. Babb A, Strand M, Uveli D, Milutinovic J, Scribner B. Quantitative description of dialysis treatment : a dialysis index. Kidney Int. 1975; 7: S23-S29.
16. Babb AL, Popovitch RP, Christopher TG, Scribner BH. The genesis of the square meter-hour hypothesis. Trans. Am. Soc. Artif. Intern. Organs. 1971; 17: 81-91.
17. Gotch F, Sargent J. A mechanistic analysis of the National Cooperative Dialysis Study. Kidney Int. 1985; 43 (suppl.40): S28-238.
18. Lopot F, Valek A. Time averaged concentration – Time Averaged deviation ; a new concept in mathematical assessment of dialysis adequacy. Nephrol. Dial. Transplant. 1998; 3: 846-848.
19. Laurent G, Charra B. The results of an 8 h thrice weekly haemodialysis schedule. Nephrol. Dial. Transplant. 1998; 13 (6): 125-131.
20. Kenley R. Home Hemodialysis : Technical and Economic Concerns (Conventional vs Daily). Fourth International Symposium on Home Hemodialysis, Nashville, 1998.
21. Arend SM, Mallat MJK, Westendorp RJW, Van der Woude FJ, Van Es LA. Patient survival after renal transplantation ; more than 25 years follow-up. Nephro. Dial Transplant. 1997; 12: 1672-1679.
22. Mun Woo Y, Jardin AG, Clark AF, MacGregor MS, Bowman AW, Macpherson SG, Briggs J.D, Junor BJR, McMillan MA, Stuart C.Rogder R. Early graft function and patient survival following cadaveric renal transplantation. Kidney Int. 1999; 55: 692-699.
23. Frei U, Schindler R, Wieters D, Grouven U, Brunkhorst R, Koch KM. Pre-transplant hypertension : a major risk factor for chronic progressive renal allograft dysfunction ? Nephrol. Dial. Transplant. 1995; 10: 1206-1211.
24.Van Loo AA, Vanholder RC, Bernaert PR, Vermassen FE, Van der Vennet M, Lameire NH. Pretransplantation Hemodialysis Strategy Influences Early Renal Graft Function. J. Ann. Soc. Nephrol. 1998; 9: 473-481.
25. Winchester JF, Rotellar C, Goggins M, Robino D, Alijani MR, Rakowski TA, Argy WP. Transplantation in peritoneal dialysis and hemodialysis. Kidney Int. 1993; 43 (Suppl. 40): S101-S105.
26. Evangelista JB Jr, Bennett-Jones D, Stewart Cameron J, Chisholm Ogg, Gwyn Williams D, Taube DH, Neild G, Rudge C. Renal transplantation in patients treated with haemodialysis and short term and long term continuous ambulatory peritoneal dialysis. B. Med J. 1985; 291: 1004-1007.
27. Vanholder R, Heering P, Van Loo A, Van Biesen W, Lambert MC, Hesse U, Van der Vennet M, Grabensee B, Lameire N. Reduced Incidence of Acute renal graft failure in patients treated with peritoneal dialysis compared with hemodialysis. Am. J. Kidney Dis. 1999; 33: 934-940.
28. Meurisse M, Albert A, Defraigne JO, Bonnet P, Honore P, Pirenne J, Henrivaux P, Mahieu P, Beaujean MA, Limet R, Jacquet N. Multiple risk factor analysis of non-immunological delayed graft function after kidney transplantation. Clin. Transpl. 1988; 2: 312-318.




