
WHEN IT IS TIME TO INITIATE DIALYSIS ?
Francisco Caravaca
E-mail: fcaravacam@senefro.org,
S. Nefrología. Hospital Universitario Infanta Cristina.
Badajoz. Spain
| DISCUSSION BOARD |
The beneficial effects that dialysis can offer to the pre-dialysis renal failure patient is one of the most basic argument for supporting our decision. On the other side, the potential complications of dialysis, and the changes in the way of life that many patients have to endure, are factors which should temper this decision.
The main goals of dialysis treatment are: to maintain patients free of uremic symptoms, to control volume overload, acid-base and electrolyte disorders, and to provide a clearance of uremic toxins enough to allow an adequate dietary protein and caloric intake. When residual renal function fails to maintain all these vital functions, then, we have a solid argument for starting dialysis therapy. The key question is whether we have to start dialysis prior to, or after the overt development of these uremic signs and symptoms.
In a very interesting opinion survey about the initiation of dialysis carried out at the ERA-EDTA Congress (Nice, 2000) [1], the majority of the participants answered that uremic signs and symptoms, along with residual renal clearances were the most important criteria for judging when to initiate dialysis. In contrast, less than 20% of participants thought that nutritional status was an important criterion to initiate dialysis. We, therefore, may infer that the majority of nephrologists in Europe take the decision to start dialysis when their patients have low renal function associated with overt uremic signs and symptoms.
Based on these facts, I would like to discuss in this presentation several points which may facilitate the election of the best timing to initiate dialysis.
AT WHICH DEGREE OF RENAL INSUFFICIENCY SEVERITY THE DETRIMENTAL EFFECT OF UREMIA BECOME MORE EVIDENT?
Symptoms associated with terminal uremia are unspecific, making it difficult to decide when to start dialysis. In recent years, the idea that severity of azotemia should be evaluated by its potential detrimental effect on nutritional status in pre-dialysis patients has gained much support [2-5].
Primarily based on this relationship between nutritional status and renal function, DOQI guidelines [6] and Canadian guidelines [7] recommend to initiate dialysis when GFR falls below 10.5 and 12 ml/min/1.73 m2, respectively. The evidence to support these guidelines is not based on firm data, but on opinion.
In order to confirm the relationship between uremic signs and symptoms, including the nutritional status, with the degree of renal insufficiency severity, we carried out a cross-sectional study in 201 pre-dialysis end-stage renal failure patients [8]. A uremic score, composed of the uremic symptoms, the subjective global assessment of nutritional status, serum albumin concentration, and protein catabolic rate normalized for ideal body weight, was taken as a clinical marker of uremic toxicity. Renal function was assessed through the arithmetic mean of creatinine and urea clearance corrected for 1.73 m2 (Ccr-Cu). The correlation that best fit Ccr-Cu with the uremic score was a cubic curve in which an ascending inflection was observed when Ccr-Cu fell below 10 ml/min/1.73 m2 (Figure 1).The results from this study suggest that Ccr-Cu is a reliable method for predicting the development of symptoms and malnutrition due to terminal uremia, irrespective of the patients characteristics. Although the results from this study do not predict irreversible consequences of uremia for delaying the initiation of dialysis when GFR falls below 10 ml/min, nephrologist should be aware that potential uremic symptoms and malnutrition become sharply more intense when GFR falls below this point, and thus, appropriate measures for preparing the initiation of dialysis (information and election of dialysis modality, vascular access, etc.) should be performed before or shortly after reaching this point of renal insufficiency severity .
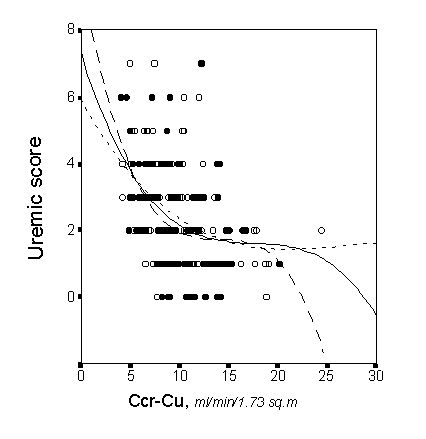
Figure 1. Correlation between the uremic score and the arithmetic mean of the creatinine and urea clearance (Ccr-Cu) corrected for 1.73 m2. Individual male (closed circles, and discontinuous line) and female patients (open circles, dotted line) can be identified in the plot.
Apart from the degree of renal insufficiency severity, the anemia and comorbidity strongly influenced on the uremic score of these study patients [8]. Figure 2 and 3 depict the partial regression lines between the uremic score and renal function for patients with different degrees of anemia severity, and the partial regression lines for patients with or without comorbid conditions. Anemia was a major determinant of malnutrition and uremic symptoms, and more than 80% of patients with malnutrition had comorbid conditions [8]. These results should lead us to the notion that not only the severity of renal insufficiency is important in determining the global health status of end-stage renal failure patients, and probably in determining their outcome, as I will discuss below.
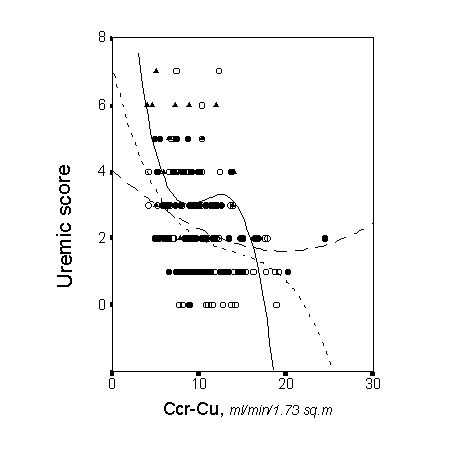
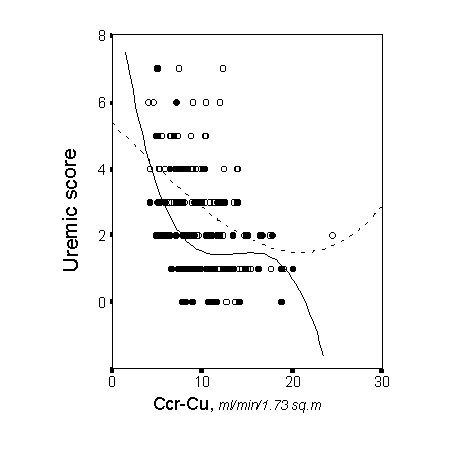
METHODS FOR ASSESSING RENAL FUNCTION IN PRE-DIALYSIS PATIENTS
When basing decisions for starting dialysis on measurements of residual renal function it is important to use appropriate techniques. The accuracy of serum creatinine as an estimate of renal function is very limited [9,10].
Based on the results of the adequacy parameters in peritoneal dialysis patients, a weekly renal Kt/V urea value of <2 Kt/V urea is largely dependent upon V (urea distribution volume), which probably causes the discrepancy between Kt/V urea and other clearances corrected for a standard body surface area. This relationship may explain potential errors in the interpretation of Kt/V urea. For instance, underweight malnourished patients usually have a Kt/V urea which tends to overestimate creatinine clearance corrected for 1.73 m2. On the contrary, a Kt/V urea value <2 In order to avoid the 24-hour urine colection for measuring the renal clearances of endogenous substances, task which is inconvenient for many patients, the use of predictive equations for estimating the GFR have been widely accepted. In 1976, Crockcroft and Gault published a prediction equation of creatinine clearance from serum creatinine, in which the age, weight and sex were included as variables [13]. Although this formula is extensively used, its accuracy to establish critical degrees of renal insufficiency is far from optimal.
The Modification of Diet in Renal Disease (MDRD) Study, a multicenter, controlled trial, evaluated the effect of dietary protein restriction on the progression of renal disease [14]. During the baseline period, GFR was measured as the renal clearance of 125I-iothalamate in 1628 patients. Using this measure of GFR as a gold standard, a prediction equation was developed, in which age, sex, race, serum creatinine, serum urea nitrogen and serum albumin concentrations were included as predictive variables [15].
This formula has been widely accepted, and it is being used in clinical investigations as the unique method for estimate the GFR [16-18]. However, an important factor which one must take into account when this formula is used in unselelected end-stage renal failure population is the fact that the population included in the MDRD study was very selected [14]. Patients older than 70 years, diabetics on insulin therapy, patients with serum creatinina over 7 mg/dl, or with proteinuria over 10 g / day, and those with other "chronic medical conditions" were not included in this study.
At present, a great proportion of patients who need to start dialysis has any of these clinical characteristics. Because MDRD formula has not been validated in these patients, the GFR obtained with this formula in unselected end-stage renal failure population should be interpreted with caution.
Due to significant differences may be obtained with most of the methods currently used for estimate the GFR, the definition of a value or range of values of GFR at which dialysis should be started is very difficult.
Perhaps, this is the most controversial point of this subject. In agreement with Prichard´s comment [1], I think that we must distinguish clearly the concepts of "earlier and later starts", and "early and later referrals". These concepts are frequently mixed in the literature, clouding the interpretation on whether the appropriate pre-dialysis care is more important over the morbidity and mortality than the specific value of GFR at which patients start dialysis.
Late-referrals patients, and, non-elective (in-hospital) initiation of dialysis have been associated with early mortality, higher comorbidity, and a greater cost of the treatment [19-22]. Late-referrals patients usually start dialysis with more severe degrees of renal insufficiency severity than the early-referrals. Infering from these observations, opinions toward an earlier control and initiation of dialysis have increasingly gained supporters.
We studied prospectively the determinants of the early mortality in dialysis of 140 end-stage renal failure patients who had been previously followed in the pre-dialysis consult [23]. Neither the follow-up time in the predialysis consult (from 1 to 46 months), nor the Ccr-Cu at the start of dialysis (7.26±1.86 ml/min/1.73 m2), were determinants of the mortality among these patients. The best determinants of the early mortality stratified for an age over or less than 65 years, was the nutritional status prior to the initiation of dialysis, which in turn was strongly associated with the comorbidity. The antecendent of cardiovascular disease influenced on the mortality, especially in patients younger than 65 years. These results suggest that there are more important causes of mortality among end-stage renal failure patients than the degree of renal insufficiency severity at the initiation of dialysis, at least in patients who have received an appropriate pre-dialysis care.
Notwithstanding, the general sense about the late-referral patients in most dialysis units [19-22], with which I completely agree, is that these patients have a higher morbidity and mortality, as well as a greater cost of treatment. Therefore, I would like to emphasize in this presentation how important is for these patients the early referral to the pre-dialysis consult.
In my experience, there are several circumstances which make the chance to start dialysis earlier and in a non-elective way more likely. These circumstances are: the overhydratation, the anemia, and the comorbidity.
End-stage renal failure patients with overhydratation are more likely to have effective volume overload than patients with less severe renal insufficiency or normal renal function [24,25].
This fact has two main implications. First, when we evaluate the GFR of an end-stage renal failure patient with volume overload, we should keep in mind that this value of GFR may be overestimated by volume overload, and we should expect a more rapid decline of renal function if reduction in volume overload is accomplished with an adequate therapy. Second, if we fail to control volume overload, the development of heart failure is a common event among these patients, which in turn may precipitate a rapid fall of GFR.
Anemia is probably the most important factor in determining the severity of symptoms and signs of end-stage renal failure [8, 26,27]. Moreover, it has a very negative impact on morbidity and mortality, being especially harmful on myocardial function [28-31].
Animal experiments showed that raising hematocrit to the normal level in rats submitted to subtotal nephrectomy resulted in accelerated progression, higher proteinuria, and higher incidence of glomerular sclerotic lesions [32,33]. The results from these studies arose major concern about the use of EPO in predialysis patients. However, EPO therapy has not been demonstrated to be nephrotoxic per se [34], or to modify the rate of progression of renal failure in humans [35-37]. Furthermore, there are several studies which suggest that EPO therapy may even improve the rate of progression of renal insufficiency, and delay the need for renal replacement therapy [38,39].
Prospective randomized studies about the effect of anemia and EPO therapy on the progression of renal failure are now difficult to carry out for ethical reasons. On the other hand, retrospective studies usually assume that all the etiologies of renal failure have the same rate of renal function decline, and this assumption is not true [40,41]. Thus, it would be interesting to analyse in future studies the effect of anemia and EPO therapy on the progression of renal failure, adjusting the results according to the different etiologic subgroups, or to the main circumstances which determine the progression of renal insufficiency at this terminal stage.
Diabetic patients have substantially more symptoms and signs of uremia than other patients when they reach end-stage renal failure [8]. Many symptoms of terminal uremia and many symptoms secondary to a long-term complicated diabetes are very similar, and they frequently overlap. Overhydratation and severe arterial hypertension are very common in this etiologic subgroup. All these factors make easier the decision to start dialysis earlier in these patients. However, there is not proof so far that this practice improves the prognosis of these patients.
Patients with myocardial diseases tolerate bad volume overload and anemia. If measures for correcting these alterations fail, dialysis should be initiated.
In general, patients with comorbid conditions have an increase risk to be exposed to nephrotoxic drugs. Radiographic contrast, antibiotics, nonsteroidal antinflamatory drugs, ACE inhibitors, or even an inappropriate use of diuretics may precipitate a rapid fall of GFR.
Severe malnutrition is a more controversial indication for early start of dialysis. Although it is nodoubt that uremia worses the nutritional status, in very few cases severe malnutrition can be attributed to uremia per se. Chronic uremia causes a slow and protracted deterioration of nutritional status. When GFR falls below 10 ml/min, this deterioration accelerates, and it is usually reversed after several months on dialysis.
When patients with GFR over 10 ml/min have severe malnutrition, comorbid conditions are behind all these cases. Whether earlier dialysis and nutritional support are able to improve the prognosis of these patients with severe malnutrition and comorbid conditions, is an unclear issue.
According to the above commented points, I would not dare to recommend a specific value of renal function which clearly defines the best timing for dialysis initiation. Alike other critical medical decisions, the right moment for starting dialysis should be based on the complex interactions of multiple factors. However, based on medical literature and on my own experience, I would recommend several points which may make this decision easier, and they may improve the outcome of end-stage renal failure patients:
CAN THE DEGREE OF RENAL INSUFFICIENCY SEVERITY AT WHICH DIALYSIS IS INITIATED INFLUENCE ON MORBIDITY AND MORTALITY?
SHOULD DIALYSIS BE INITIATED EARLIER IN ANY SPECIAL CASE?
CONCLUSIONS
REFERENCES
1. Ledebo I, Kessler M, van Biesen W, et al. Initiation of dialysis-opinions from an international survey: Report on the Dialysis Opinion Symposium at the ERA-EDTA Congress, 18 September 2000, Nice. Nephrol Dial Transplant 2001, 16: 1132-1138
2. Hakim RM, Lazarus JM: Progression of chronic renal failure. Am J Kidney Dis 1989; 14: 396-401
3. Modification of Diet in Renal Disease Study Group (prepared by Kopple JD, Berg R, Houser H, Steinman TI, Teschan P): Nutritional status of patients with different levels of chronic renal failure. Kidney Int 1989; 36 (suppl 27): S184-S194
4. Ikizler TA, Greene J, Wingard RL, Parker RA, Hakim RM: Spontaneous dietary protein intake during progression of chronic renal failure. J Am Soc Nephrol 1995; 6: 1386-1391
5. Hakim RM, Lazarus JM: Initiation of dialysis. J Am Soc Nephrol 1995; 6:1319-1328
6. National Kidney Foundation. Dialysis Outcomes Quality Initiative: Initiation of dialysis. Am J Kidney Dis 1997; 30 (suppl 2): S70-S73
7. Churchill DN, Blake PG, Jindal KK, Toffelmire EB, Goldstein MB: Clinical practice guidelines for initiation of dialysis. J Am Soc Nephrol 1999; 10 (suppl 13): S289-S291
8. Caravaca F, Arrobas M, Pizarro JL, Sanchez-Casado E. Uraemic symptoms, nutritional status and renal function in pre-dialysis end-stage renal failure patients. Nephrol Dial Transplant 2001, 16: 776-782
9. Levey AS. Measurement of renal function in chronic renal disease. Kidney Int 1990; 38: 167-184
10. Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: New insights into old concepts. Clin Chem 1990; 38: 1933-1953
11. Mehrotra S, Saran R, Moore HL, et al. Toward targets for initiation of chronic dialysis. Perit Dial Int 1997; 17: 497-508
12. Saran R, Moore HL, Mehrotra S, Khanna R, Nolph KD: Longitudinal evaluation of a renal Kt/V (urea) of 2.0 as a threshold for initiation of dialysis. ASAIO J 1998; 44: 677-681
13. Crockcroft D, Gault M. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31-41
14. Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Eng J Med 1994, 330:877-884
15. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, for the Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 1999; 130: 461-470
16. Obrador GT, Arora P, Kausz AT, Ruthazer R, Pereira BJG, Levey AS. Level of renal function at the initiation of dialysis in the U.S. end-stage renal disease population. Kidney Int 1999; 56: 2227-2235
17. Kausz AT, Obrador GT, Arora P, Ruthazer R, Levey AS, Pereira BJG. Late initiation of dialysis among women and ethnic minorities in the United States. J Am Soc Nephrol. 2000; 11: 2351-2357
18. Moist LM, Port FK, Orzol SM, et al. Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol 2000; 11: 556-564
19. Ratcliffe PJ, Phillips RE, Oliver DO: Late referral for maintenance dialysis. BMJ 1984; 288: 441-443
20. Jungers P, Zingraff J, Albouze G, et al. Late referral to maintenance dialysis: detrimental consequences. Nephrol Dial Transplant 1993; 8:1089-1093
21. Khan IH, Catto GRD, Edward N, MacLeod AM: Death during the first ninety days of dialysis: a case control study. Am J Kidney Dis 1995; 25: 276-280
22. Holland DC, Lam M. Suboptimal dialysis initiation in a retrospective cohort of predialysis patients--predictors of in-hospital dialysis initiation, catheter insertion and one-year mortality. Scand J Urol Nephrol 2000;34:341-347
23. Caravaca F, Arrobas M, Pizarro JL, et al. Predictores de la moralidad precoz en diálisis. Nefrología 2001; 21: 274-282
24. Koomans HA, Geers AB, Mees EJ Plasma volume recovery after ultrafiltration in patients with chronic renal failure. Kidney Int 1984;26:848-854.
25. Koomans HA, Braam B, Geers AB, Roos JC, Dorhout Mees EJ. The importance of plasma protein for blood volume and blood pressure homeostasis. Kidney Int 1986;30:730-735
26. Holland DC, Lam M. Predictors of hospitalization and death among pre-dialysis patients: a retrospective cohort study. Nephrol Dial Transplant 2000;15:650-658
27. Revicki DA, Brown RE, Feeny DH, et al. Health-related quality of life associated with recombinant human erythropoietin therapy for predialysis chronic renal disease patients. Am J Kidney Dis 1995;25:548-554
28. Fink J, Blahut S, Reddy M, Light P. Use of erythropoietin before the initiation of dialysis and its impact on mortality. Am J Kidney Dis 2001;37:348-355
29. Portoles J, Torralbo A, Martin P, Rodrigo J, Herrero JA, Barrientos A. Cardiovascular effects of recombinant human erythropoietin in predialysis patients. Am J Kidney Dis 1997;29:541-548
30. Hayashi T, Suzuki A, Shoji T, et al. Cardiovascular effect of normalizing the hematocrit level during erythropoietin therapy in predialysis patients with chronic renal failure. Am J Kidney Dis 2000;35:250-256
31. Silverberg D, Blum M, Peer G, Iaina A. Anemia during the predialysis period: A key to cardiac damage in renal failure. Nephron 1998;80:1-5
32. Garcia DL, Anderson S, Rennke HG, Brenner BM. Anemia lessens and its prevention with recombinant human erythropoietin worsens glomerular injury and hypertension in rats with reduced renal mass. Proc Natl Aca Sci USA 1988;85:6142-6146
33. Lafferty HM, Garcia DL, Rennke HG, at al. Anemia ameliorates progressive renal injury in experimental DOCA-salt hypertension. J Am Soc Nephrol 1991: 1:1180-1185
34. Bellizzi V, Sabbatini M, Fuiano G, et al. The impact of early normalization of haematocrit by erythropoietin on renal damage in remmant kidney model. Nephrol Dial Transplant 1998; 13:2210-2215
35. Roth D, Smith RD, Schulman G, et al. Effects of recombinant human erythropoietin on renal function in chronic renal failure predialysis patients. Am J Kidney Dis 1994;24:777-784
36. Van Geet C, Van Dyck M, Proesmans W. Subcutaneous recombinant erythropoietin in preterminal renal insufficiency. Eur J Pediatr 1994;153:129-132
37. Mitwalli A, Abuaisha H, al Wakeel J, et al. Effectiveness of low-dose erythropoietin in predialysis chronic renal failure patients. Nephrol Dial Transplant 1993;8:1085-1088
38. Kuriyama S, Tomonari H, Yoshida H, Hashimoto T, Kawaguchi Y, Sakai O. Reversal of anemia by erythropoietin therapy retards the progression of chronic renal failure, especially in nondiabetic patients. Nephron 1997;77:176-185
39. Jungers P, Choukroun G, Oualim Z, Robino C, Nguyen AT, Man NK. Beneficial influence of recombinant human erythropoietin therapy on the rate of progression of chronic renal failure in predialysis patients. Nephrol Dial Transplant 2001;16:307-312
40. Jungers P, Hannedouche T, Itakura Y et al. Progression rate to end-stage renal failure in non-diabetic kidney diseases: a multivariate analysis of determinant factors. Nephrol Dial Transplant 1995; 10: 1353-1360
41. Rottey S, Vanholder R, De Schoenmakere G, Lameire N. Progression of renal failure in patients with compromised renal function is not always present: evaluation of underlying disease. Clin Nephrol 2000;54:1-10.