
RENAL TRANSPLANTATION IN CHILDREN LESS THAN 6 YEARS OF AGE
Clotilde Druck Garcia
E-mail: cdgarcia@terra.com.br
Complexo Hospitalar Santa Casa
Fundação Faculdade Federal de Ciências Médicas de Porto Alegre - Brazil.
| DISCUSSION BOARD |
key words: pediatric renal transplant, basiliximab, vessel thrombosis
Pediatric Renal Transplant Team:
Viviane Barros, Valter Duro Garcia, Santo Pascoal Vitola, Eduardo Didone, Enilde Guerra, Fabian , Guido Cantisani, Maria Lucia Zanoteli, Paulo Mota, Vitor Rocha, Jorge Neumann
Introduction
A successful kidney transplant remains the most effective renal replacement therapy for children with end-stage renal failure (1,2). During the last two decades renal transplantation has become routine treatment for uremic children. However, there are many technical, immunologic, metabolic and psychological factors that make the results in children and adolescents different from those in adults.(2b).A major difference between children and adults relates to the causes of ESRD; structural abnormalities of urinary tract and focal segmental glomerulosclerosis with its high risk with recurrence predominates in pediatric patients. Pediatric renal transplantation presents a number of challenges, especially in the younger age group. Graft and patient survival were often reported to be not so good in young recipients compared with older children and adults, but the results have been improved (3,4,5).
The objective of this paper is to review the peculiarities of renal transplantation in small children and to report our single-center experience with renal transplantation in children less than 6 years of age.
Patients and Methods
From March 1977 to September 2001, a total of 207 pediatric renal transplants were performed in our institution. Twenty-three renal transplants were performed in 22 children aged 5 years and younger; the first one was performed in March 1989. One child required a second transplant. These patients were operated on and cared for by the same pediatric renal transplant team. All grafts were placed extra-peritoneally. Venous anastomosis of the donor renal vein was performed with caval vein or iliac vein and the arterial anastomosis with aorta or common iliac artery. In the last years the vascular anastomosis were made only to aorta and caval vein in this special group of small children. No anticoagulation therapy was administered to the patients. The ureter was preferentially implanted using Gregoire thecnic.
The mean (range) age of the recipients at transplant was 3.14 (2.0-5.9) years old and the mean (range) body weight was 13.9 (9.5-19.5) kg. Six of the transplantations were performed from 1989 to 1993 and the remaining 17 from 1994 to 2001.
The diseases leading to renal failure in these 22 children included mainly congenital urological disorders (n=8), hemolytic uremic syndrome (n=4), congenital nephrotic syndrome (n=3), glomerulonephritis (n=3), nephrosialidosis (n=1), ischemic cortical necrosis (n=1), glomerulocystic disease (n=1) and one unknown disease.
Transplantation was performed as soon as possible, after stabilization of medical issues, optimization of nutrition, and reaching of desired weight of at least 8 kg, but preferably of 10kg. Twenty-two patients were on dialysis before transplant, 19 on CAPD and 3 in hemodialysis. One transplant was pre-emptive.
Kidneys from living-related donors (17 parents and 2 grandmothers) were used in 19 patients (83%) and from cadaveric donors in 4 (17%). The mean (range) donor age was 26 (6-55) years old. All grafts were placed extra-peritoneally. Venous anastomosis of the donor renal vein was performed with caval vein or iliac vein and the arterial anastomosis with aorta or common iliac artery. In the last years the vascular anastomosis were made only to aorta and caval vein in this special group of small children. No anticoagulation therapy was administered to the patients.
Primary immunosuppressive therapy consisted of prednisone, azathioprine and cyclosporine. Since 1999, mycophenolate mofetil (MMF) has been used instead of azathioprine and basiliximab as induction therapy. Cyclosporin and later Neoral have been used initially 8-14 mg/kg/dose each 12 hours. Doses were gradually tapered to achieve Cmax 800-1000ng/l, at 6 month, and 600-800ng/l after 6th month. Cmax appears to be a more-suitable measure of exposure to CSA than Cmin. Prediction of Tmax from the age of the child may help to overcome the problem of when to collect blood for peak levels.(6) In this young group the Cmax is around 1 to 2 hours. Tight immunusuppressive management was maintained with frequent blood drug-level monitoring (monthly).
MMF was used 600 mg/m2/dose each 12 hours. Basiliximab (12 mg/m2/dose maximun 20 mg/dose)
was given immediately before transplantation and again on fourth day.
The mean follow-up after transplantation was 34(6-84) months. The estimated glomerular filtration rate (GFR) was calculated using the Formula of Schwartz. The estimation of patient and graft survival was obtained by the Kaplan-Meier method.
Pre transplant management
The 2 patients with obstructive uropathy and one with huge vesico ureteral reflux were submitted to urological surgery prior transplant.
The recommended immunization were performed in all. They didn’t receive varicella vaccine.
All of them, except 2 were in nasogastric nutrition, and we wait at minimum of 9kg to submit for transplant..
Results
Patient and Graft Survival
The overall patient survival rate at 1 and 5 years was 95%; 1 and 5 year graft survival was 78% and 58%, respectively (table1).
Ten patients (43%) had one or more rejection episodes, 4 were steroid resistant, rescued with OKT3 (n=1) and tacrolimus (n=2). All patients recovered renal function, except two, one had also renal artery thrombosis. This patient died on sepsis, and this was the only death in this series.
The last 5 patients received basiliximab as part of the induction therapy, acute rejection did not occur in any of them.
There were 7 (30%) graft losses: 3 due to renal artery thrombosis (one immediately after transplant, one associated with a severe acute rejection and one was secondary to angioplasty for graft arterial stenosis at the site of vascular anastomosis); 1 acute rejection, 1 due to renal vein thrombosis; 2 due to chronic rejection (1 non-compliant family).
Immunosuppression
Triple immunosuppressive therapy with cyclosporine, azathioprine and prednisone was used in 15 patients. Since 1999, MMF was added to the protocol and was used in 8 children (6 primarily and 2 as conversion). Basiliximab was used as induction therapy in the last 5 patients. Five patients used tacrolimus, one as primary immunosuppression, 2 as rescue therapy for corticoresistant rejection, and 2 converted for chronic rejection.
The estimated GFR was 81+20 ml/min at the 1st year, 65+21+ ml/min at the 3rd year and 54+19 ml/min at the 5th year (table 1).
CMV disease was observed in one patient. Cytomegalovirus prophylaxis was not used. We follow the patients with antigenemia weekly in the first 3 months. Two patients had varicella. No case of malignancy or lymphoproliferative disorder was detected.
Vesico-ureteric junction obstruction occurred in 1 patient and was successfully treated with catheter placement.
At the time of transplantation the mean height Z score was –1,9 (–3.89 to 0.1). The greatest increase of height Z score was observed during the first 2 years
(-1.2± 0.9 and -1.1± 1.0, respectively 1st and 2nd year). At 5th year Z score was –1.5 (table 1).
|
Time after transplant (years) |
Graft survival rate (%) |
Renal function (ml/min)a (n) |
Z score for height (n) |
|
1 |
77.4+8.9 |
81± 20 (10) |
-1.2± 0.9 (11) |
|
2 |
77.4+8.9 |
65± 18 (7) |
-1.1± 1.0 (9) |
|
3 |
67.8+11.9 |
66± 21 (5) |
-1.6± 1.6 (5) |
|
4 |
58.1± 13.6 |
51± 22 (5) |
-1.7± 1.0 (5) |
|
5 |
58.1± 13.6 |
54± 18 (5) |
-1.5± 1.2 (5) |
Discussion
Transplantation in children less than 6 years of age accounted 14% of all pediatric transplants performed in our unit. In our institution the pediatric transplant activity initiated in 1977, but only in 1989 we did the first transplant in small children. This progress was achieved after the experience and practice of CAPD in this special group.
Donors:
The majority (86%) of transplants were performed with living related donors. Although good results have also been reported with use of cadaveric donors (1,6,7), grafts of living donors show early immediate function and a low rate of surgical complications (2,8,9), The child’s family is encouraged to identify potential living related donors, including grandparents. We used 2 grandmothers as donors (40 & 55 years old), without complications.
Within the infant group, the best results are obtained with adult-sized kidneys(10,11,12). Kidneys from cadaveric donors aged < 5 years provide the poorest graft survival in children, as much as 30 % lower than adult-sized kidneys (13, 14). Although adult kidneys provide better graft survival in infants, there is an associated greater incidence of acute tubular necrosis, graft thrombosis, and primary graft non-function. These problems have been generally attributed to marked discrepancy in size between the adult kidney and the infant recipient. (12).
Technical issues
Vessel thrombosis was the most frequent cause of graft loss in our patients, in agreement with other studies (5,7). There were 3 graft losses (2 patients) due to renal artery thrombosis and one with venous thrombosis. The careful observation of technical factors suggested by Najarian (2), Rosenthal (9) and Salvatierra (1) such as surgical technique and aggressive fluid therapy improved our results and we lost only 2 grafts from non-immunological cause in the last 9 years. To prevent graft thrombosis, primary nonfunction, and a low postoperative ATN is important to have a perfect vessels anastomosis(12). The venous anatomosis is performed between the donor renal vein and distal vena cava or right iliac vein and end-to-side arterial anastomosis is then done between the donor renal artery and the distal aorta or common iliac artery (2). Redundancy of any of these vessels can result in kinking, which would predispose the patient to graft thrombosis. Because the adult-sized kidney will occupy a good part of the right abdomen, there is little space for the renal vessels between the graft and the infant’s aorta and vena cava. Furthermore, the closure of the abdominal wound would further juxtapose the adult-sized kidney against the infant’s aorta and vena cava. Because all of these, the transplanted renal artery and vein should be shorten to prevent even the slightest redundancy after wound closure. The anastomosis should be perfect, with a perfect positioning of the donor kidney blood vessels, and protection against ATN and technical problems. To avoid delayed graft function and ATN improve the long term results. (10,11,12) To achieve this, it’s necessary also to have maximum intravascular volume. Large kidneys sequester a large percentage of circulating blood volume and cardiac output.
Before reperfusion of the kidney, the infant’s CVP is raised to 15 to 18 cm of saline solution, albumin 5% and/or packed red blood cells. It is very important during reperfusion to maintain adequate blood pressure and filling pressures via volume expansion. Mannitol and furosemide are infused as the anastomosis completed (2). It is necessary to continue aggressive early postoperative fluid resuscitation in infants and to maintain, most small children, on postoperative mechanical ventilation for 1 to 2 days to obviate the need for fluid restriction at this critical time because of the potential for pulmonary edema.(Fig1). The need to maintain an optimum intravascular volume does not cease after the immediate perioperative period. The children received aggressive nasogastric fluid supplementation (total of 2500ml/m2/dia) until they can drink this volume.(14)(Figure 2)
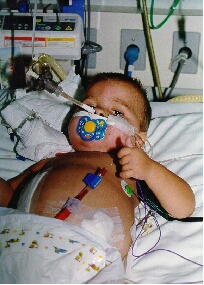 | 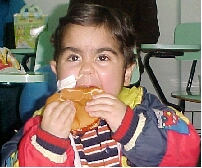 |
Figure 1: OB, 2 years old, in mechanical ventilation because aggressive fluid hydration | Figure 2: DP, 2 years old, 3 months post transplant, on nasoenteral tube fluid supplementation |
Immunosuppression , Rejection and Survival
We are using now as primary immunosuppression cyclosporin (syrup), MMF and prednisone and induction therapy with basiliximab. We are not given Tacrolimus to the young population with difficult to swallow pills. Many centers dissolve Tacrolimus in water and calculate the dose. Tacrolimus was used in this series as rescue in patients with acute or chronic rejection.
Acute rejection occurred in 41% of patients, similar to other series (7). The use of basiliximab decreases the incidence of rejection (15,16), as occurred in our experience in this population (17).
The patient survival was 95 % in the 1st and 5th year. The graft survival was 77% and 58% in the 1st and 5th year respectively. These results are poorer when comparing with older children. Salvatierra has better results with smaller children, 100% 2year graft survival (12). The result of North Italy Transplant Program of graft survival was 74.5% and 70.5% in the 1st and 5th year, using cadaveric donors (7).
Graft function, Growth and Development
The graft function in these patients, has decreased with time. The same happened with others as Salvatierra had published, due to decreased intravascular volume to perfuse adult kidneys in these small patients (10).
Growth improved in our patients but in general they did not reach the normal stature for age, as already observed (5,8). Growth hormone is effective and safe in transplanted children (18). Treatment with growth hormone is very expensive only one child is using now, with good results and without rejection episodes.
None of these children showed any sign of psychomotor retardation and the children above 6 years old are attending ordinary school.
We conclude that renal transplantation is the best chance for uremic young children to achieve adequate development and a normal life. We recommend renal transplant for small children as soon they achieve good clinical conditions, preferable with living related donor. The use of new immunosuppressors, as IL2 receptors, maximun hydration and special care in vessels anastomosis are important to the success of renal transplantation in small children.
References
2b. Ettenger RB. Children are different: the challenges of pediatric renal transplantation. Am J Kidney Dis 20:668-672, 1992
Correspondence address: CD Garcia
Rua Correa Lima 1493
90850-250 Porto Alegre – Brazil
e-mail: cdgarcia@terra.com.br