
Carolina Frías, Ricardo Lauzurica.
| DISCUSSION BOARD |
Epstein-Barr virus (EBV) infection in transplant patients has been associated with the development of posttransplant lymphoproliferative disorders (PTLD) whose prognosis is usually poor. Several factors can account for PTLD development: the type of organ transplanted, type, duration and degree of immunosuppressive therapy, age of recipient and EBV-seronegativity prior to transplantation, therapy with antilymphocyte antibodies both for induction and/or as therapy for acute rejection and CMV mismatch between donor and recipient.
PTLD therapies have included: tapering or withdrawal of immunosuppression, use of antivirals (acyclovir, ganciclovir), γ-IFN, anti CD-21, anti CD-24 and other monoclonal antibodies, surgery and chemotherapy. Early therapy may improve PTLD prognosis, which emphasizes the relevance of a rapid diagnosis.
Usefulness of serological techniques for diagnostic purposes is limited because serological response from transplant recipients is defficient. The polymerase chain reaction (PCR) technique has been used for detecting and quantifying EBV in clinical samples. An association has been found between high EBV load values in blood samples and PTLD, but its value as an early diagnostic marker for PTLD development as well as for monitoring the efficacy of therapy are still unconfirmed. It has been suggested that EBV load assessment in adult transplant recipients may have clinical value for: early PTLD diagnosis allowing prompt therapy, monitoring the efficacy of PTLD therapy and in the differential diagnosis of PTLD and acute rejection that have opposite therapeutical approaches (reduction vs. increase in immunosuppression).
Our aims were to set-up and standardize a PCR semiquantitative method for assessing EBV load in clinical samples and to use it for prospectively follow-up EBV load in blood and saliva samples from adult kidney transplant recipients; to analyze EBV load in case of clinical features and according to the type of immunosuppression.
Sixty-five patients who underwent kidney transplantation (KT) at the Hospital Universitari Germans Trias i Pujol, Badalona between July 1997 and June 2000 were prospectively monitored. Saliva and blood samples were collected just before transplantation, every 15 days between the first three months, at 4, 5, 6, 9, 12 months after transplantation and once a year thereafter. Samples were also collected in case of clinical features: fever of infectious origin not bacterial and acute rejection (AR).
Assessment of EBV load in clinical samples was accomplished by a PCR semiquantitative method and detection of amplification products was carried out by DNA-enzymeimmunoassay (DEIA) (Sorin Biomedica, Saluggia, Italy). Previously, it was necessary to establish the dynamic range between the PCR technique and the DEIA method and to build a semilogarithmic reference curve. By plotting the absorbance values obtained by the DEIA method in the aforementioned reference curve the EBV DNA copy number were obtained. For PBMC samples, results were expressed as EBV DNA copy number/75.000 PBMC and for saliva samples as EBV DNA copy number/5 μl de saliva.
The sensitivity of the semiquantitative PCR for EBV detection was 5-10 copies of EBV DNA.
Sixty-five KT recipients were prospectively studied, the mean follow-up period was 20.4 months (range 3-36 months), 16 patients were followed between 3 months-1 year, 23 patients between 1-2 years, and 26 patients between 2-3 years. According to immunosuppressive therapy 60% of patients received tacrolimus (Fk), 31% cyclosporin A (Cy A), and 31% of patients were on antilymphocyte antibodies. A total of 1087 samples were tested: 633 blood and 454 saliva. All patients were EBV-seropositive prior to kidney transplantation.
EBV load in saliva samples was highly variable among KT patients. However, a pattern of proggressive increase both in the rate of patients shedding EBV and in the mean value of EBV DNA copy number was observed after the second week post-transplantation, reaching peak values between the third and sixth month and decreasing afterwards. Highest EBV values were found in patients on antilymphocyte antibodies both for induction and for AR therapy (Figure 1.). There was no relationship between EBV load in saliva and clinical features.
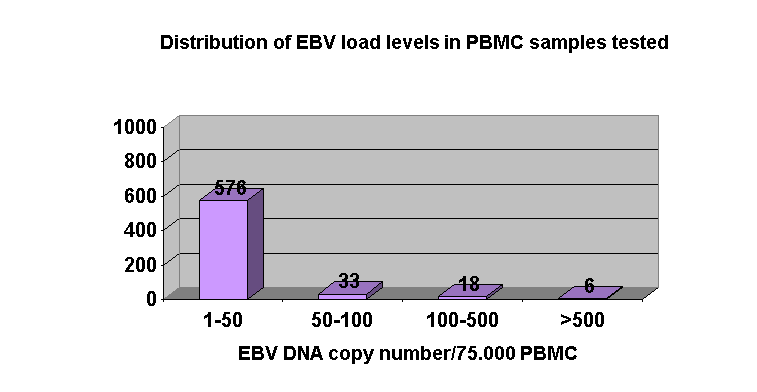
Regarding blood samples results, in 26 KT patients EBV load was always below 50 EBV DNA copies/75.000 PBMC. In 29 patients, although mean EBV load levels were between 25-50 EBV DNA copies/75.000 PBMC throughout the study period, higher load values were detected incidentally without apparent clinical features, with the exception of one patient (Figure 2.). There was no relationship between EBV load values in saliva and PBMC samples.

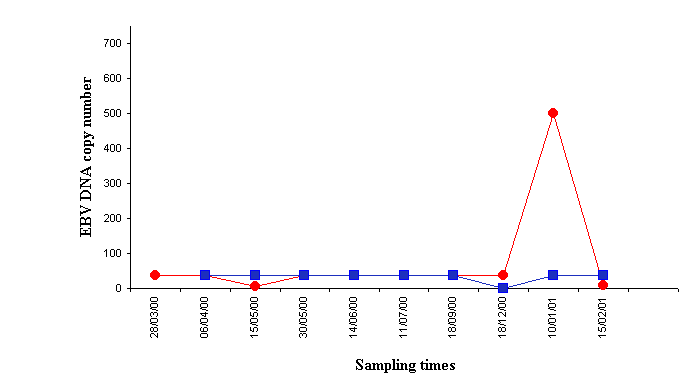
As far as clinical features, one of the 65 KT patients developed a primary brain B-cell lymphoma nine and a half months after kidney transplantation, which means an incidence of 1.3%. In that patient, the lymphoma biopsy sample was EBV positive by PCR technique. Analysis of EBV load in blood samples showed a marked increase at the time of PTLD diagnosis, followed by a decrease to levels similar to those found before lymphoma diagnosis five weeks after therapy was started, which consisted of withdrawal of immunosuppression, chemotherapy and acyclovir (Figure 3.).
In addition, there were 27 fever episodes in 19 KT and 15 AR episodes in 14 patients which were treated with methyl-prednisolone pulses. There was no relationship between EBV load in PBMC or saliva and fever episodes. When EBV load before and after AR was compared, an increase in EBV DNA levels was detected in 2 cases before AR, whereas in 4 patients EBV DNA levels increased up to 250-500 EBV DNA copies/75.000 PBMC after AR therapy with corticoids.
EBV load was assessed in 11 transplant patients with CMV infection (8 primoinfections and 3 reactivations). Diagnosis of CMV infection was carried out by pp65 detection in PBMC (antigenemia test) and CMV IgG detection in serum samples (seroconversion). All CMV-seronegative patients prior to transplantation receiving the graft from a CMV-seropositive donor received prophylaxis with ganciclovir for 1 month. Therapy for CMV infection consisted in ganciclovir for 3 months. In 5/11 KT patients with CMV infection (45%) there was evidence of EBV reactivation. Nevertheless, in none of these patients variations in EBV load neither in saliva nor in PBMC were detected. Therapy with ganciclovir had an effect on EBV DNA in saliva but not in PBMC. Type of immunosuppressive therapy (cyclosporin A vs. Fk) did not have an effect on EBV load.
According to our results, there was no relationship between EBV load in saliva and PBMC or between EBV load in saliva and clinical features. Therefore, we believe that saliva samples are of little value for prospective monitoring of EBV levels in transplant recipients.
Positive predictive value of high EBV DNA levels ³ 500 EBV DNA copies/75.000 PBMC is low since only 1/6 KT patients with viral load levels ³ 500 EBV DNA copies/75.000 PBMC developed a PTLD. In contrast, the negative predictive value of EBV levels below 50 EBV DNA copies/75.000 PBMC seems to be high just as any KT patient with EBV levels <50 EBV DNA copies/75.000 PBMC developed PTLD.
In asymptomatic patients with EBV DNA levels in PBMC between these two values (50-500 EBV DNA copies/75.000 PBMC), a more close-up follow-up is needed to assess the risk of developing a PTLD.
In our experience, a decrease in EBV load in patients with PTLD may also be useful as a marker for the efficacy of therapy. EBV monitoring in transplant patients may help to distinguish PTLD from AR, since only in 13.3% of patients with AR there was an increase of EBV load prior to the AR episode.