
BASIS FOR HYPERNATREMIA IS REVEALED BY A TONICITY BALANCE BUT NOT BY AN ELECTROLYTE-FREE WATER BALANCE
Ana PCP Carlotti1 MD, Desmond Bohn2 MB, Mogamat Razeen Davids3MD, J-P Mallie4 MD, Mitchell L. Halperin5 MD 1 Department of Pediatrics, Universidade de Sao Paulo, Ribeirao Preto, Sao Paulo, Brazil
E-mail: mitchell.halperin@utoronto.ca
2Department of Critical Care Medicine, Hospital for Sick Children, Toronto, Canada
3Nephrology Unit and Department of Internal Medicine, University of Stellenbosch, Cape Town, South Africa
4Renal Division, Hôpital d’Enfants, CHU-Nancy, France
5Renal Division, St. Michael’s Hospital, University of Toronto, Canada
| DISCUSSION BOARD |
INTRODUCTION
Rose and fellow investigators [1-3] highlighted two points to improve our understanding of the implications of a change in the plasma sodium (Na+) concentration (PNa). First, he emphasized that alterations in body water were largely responsible for a change in the PNa. Second, he cautioned against using osmole-free water terms to indicate whether there would be a change in cell volume. The rationale was that urea is sufficiently permeable to have an equal concentration in the intracellular fluid (ICF) and extracellular fluid (ECF) compartment. Therefore the concentration of urea does not influence the ICF volume. Thus he recommended that mass balance should be calculated in electrolyte-free water (EFW) terms (Figure 1) [1-3]. In this analysis, an imaginary calculation is performed where infusions and urine are divided into two compartments, isotonic saline (same PNa + potassium (K+) concentration (PK) as in the patient, and the EFW.
FIGURE 1: CALCULATIONS BASED ON THE URINE COMPOSITION
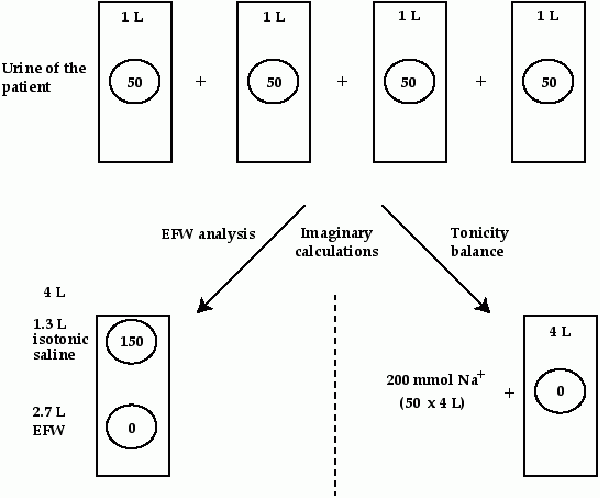
For details, see text. The concentration of Na+ in each liter is depicted within the circles. The patient excreted 4 liters of urine and 200 mmoles of Na+ + K+ with a Na+ + K+ concentration of 50 mmol/l. To calculate either an EFW or tonicity balance, one must know the urine volume and measure the urine Na+ + K+ concentrations. One must also know the composition and volumes of the infusions to calculate balances. |
An EFW balance predicts the degree of change in the PNa. Nevertheless, it does not reveal the basis for this change in PNa nor does it help to decide what is the correct therapy for hypernatremia or hyponatremia. A better way to determine why the PNa changed is to calculate a tonicity balance where all inputs and outputs are also divided into two components, total volume of water and Na+ + K+—each is analyzed separately [3-5]. Mass balance for Na+ plus K+ rather than just Na+ must be included because Na+ may enter cells in conjunction with the exit of K+ [6].
When considering Na+ + K+ in isolation, for every mmol retained per liter of total body water, the rise in PNa will be 1 mmol/l. Similarly, a gain of 1 liter of water, when considered in isolation should lower the PNa by the formula: PNa times (1/total body water).
The following three items are identical when calculating an EFW and a tonicity balance. First, the same measurements are needed to calculate each balance. Second, the initial body weight and an estimate of body composition are needed to deduce total body water. Third, both forms of external balance will not indicate why the PNa changed if the cause was due to a shift of water across cell membranes (e.g., a gain of particles restricted to the ECF compartment such as hyperglycemia [7]) or in the ICF compartment (e.g., during a seizure [8]).
Case: A craniopharyngeoma was resected today from a 14-year-old boy (weight 40-kg, total body water 24 liters). Intravenous therapy consisted of 3 liters of isotonic saline. His total urine volume was 4 liters, urine osmolality was 120 mOsm/kg H2O, and the urine [Na+ + K+] was 50 mmol/l. Over this period, his PNa rose from 140 to 157 mmol/l (Figure 2). This excretion of 200 mmoles of Na+ + K+ can be thought of as the excretion of 1.3 liters of isotonic saline and 2.7 liters of EFW (Figure 1). Because the input did not contain EFW, there was a negative balance of 2.7 liters of EFW.
FIGURE 2: COMPARISON OF AN EFW AND A TONICITY BALANCE IN THE ILLUSTRATIVE CASE
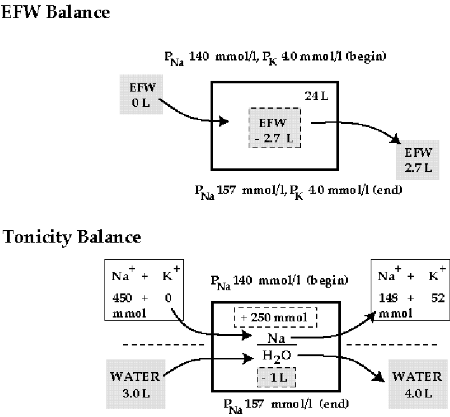 The large darker rectangles represent total body water with the PNa and PK measured at the beginning and end of the period of observation shown on the top and bottom of this rectangle, respectively. The EFW balance is shown in the dashed, shaded box inside the rectangle in the top portion of the figure. The tonicity balance is shown in the bottom portion of the figure. The quantities of Na+ + K+ infused and excreted are shown without shading above the dashed lines whereas the volumes of water infused and excreted are shown in shading below the dashed lines. Mass balance for Na+ + K+ and water are shown in dashed boxes inside the rectangle. There was a net positive balance of 250 mmol of Na+ + K+ and negative balance of 1 liter of water.
|
Comments: A clinical diagnosis of central diabetes insipidus was established because he had hypernatremia and a large water diuresis. Moreover, his urine output declined and his urine became hypertonic after vasopressin was given. The negative balance of 2.7 liters of EFW (Figure 2 top) allows one to verify that his PNa rose. If an EFW balance were used to design therapy, its aim would be to induce a positive balance of 2.7 liters of EFW, but there are many different ways to accomplish this (Table 1).
|
The PNa rose from 140 to 157 mmol/l in each setting. The only difference is the volume of isotonic saline infused over the time period of observation. In all three settings, there is a negative balance of 2.7 liters of EFW. Nevertheless, the goals of therapy to correct the hypernatremia were clear only after a tonicity balance was calculated. Illustrative case
Case If IV was 4 liters of isotonic saline and the output was unchanged
Case if no IV was administered and the output was unchanged
|
A tonicity balance also predicts the rise in his PNa (Figure 2 bottom). In addition, it provides reliable information about its basis—a gain of 250 mmol of Na+ with a water deficit of 1 liter. Hence the goals of therapy are now clear—create a negative balance of 250 mmol of Na+ (+ Cl-) along with a positive balance of 1 liter of water. This therapy will correct his hypernatremia and return both the ICF and ECF compartment volumes to normal.
The advantage of a tonicity as compared to an EFW balance is even more evident if we changed the volume of isotonic saline infused in our case. First, we could change the volume of isotonic saline infused to 4 instead of 3 liters; second, we could have no intravenous therapy (Table 1). Because there is no EFW administration and losses are unchanged in both of these hypothetical settings, the negative balance for EFW would still be 2.7 liters. Therefore the 17-mmol/l rise in natremia would be identical [2], but its basis would be different and revealed only by a tonicity balance (Table 1).
Obviously, the goals of therapy must be different in each of these examples despite the fact that the negative balance of EFW and rise in PNa were identical. The goals of therapy in the first hypothetical example are to create a negative balance for Na+ + K+ of 400 mmol and a nil balance of water. The objective in the second hypothetical example is to create a positive balance of 200 mmol of Na+ + K+ and 4 liters of EFW (Table 1).
Concluding remarks: Calculating an EFW balance is not an error, but it only reveals part of the truth. As indicated in Table 1, an EFW balance can be misleading with respect to understanding why there was a change in PNa and of greater importance, what the goals for its therapy should be. In contrast, a tonicity balance, with its separate analyses for water and Na+ + K+, provides a simple and reliable way to explain the basis for both the development of a change in the PNa and its therapy.
REFERENCES
1. Goldberg M: Hyponatremia. Med Clin North Am 65:251-269, 1981.
2. Rose BD: New approach to disturbances in the plasma sodium concentration. Am J Med 81:1033-1040, 1986.
3. Mallie J-P, Bichet DG, Halperin ML: Effective water clearance and tonicity balance: The excretion of water revisited. Clin Invest Med 20:16-24, 1997.
4. Carlotti APCP, Bohn D, Mallie J-P, et al.: Tonicity balance and not electrolyte-free water calculations more accurately guides therapy for acute changes in natremia. Intensive Care Med 27:921-924, 2001.
5. Halperin ML, Goldstein MB: Fluid, Electrolyte and Acid-Base physiology; a problem-based approach Philadelphia, W.B. Saunders, 1998, p.
6. Edelman IS, Leibman J, O'Meara MP, et al.: Interrelations between serum sodium concentration, serum osmolarity and total exchangeable sodium, total exchangeable potassium and total body water. J Clin Invest 37:1236-1256, 1958.
7. Roscoe JM, Halperin ML, Rolleston FS, et al.: Hyperglycemia-induced hyponatremia: metabolic considerations in calculation of serum sodium depression. CMA Journal 112:452-453, 1975.
8. Welt LG, Orloss J, Kydd DM, et al.: An example of cellular hyperosmolarity. J Clin Invest 29:935-939, 1950.