
NEW INSIGHTS INTO THE RENAL FAILURE ASSOCIATED WITH ATHEROSCLEROTIC RENOVASCULAR DISEASE (ARVD)
Philip A Kalra FRCP MD
Consultant Nephrologist and Honorary University Lecturer
Department of Renal Medicine, Hope Hospital,
Salford, England
| DISCUSSION BOARD |
Epidemiology of ARVD
ARVD and other vascular disease
Atherosclerotic renovascular disease (ARVD) is very common and it is encountered in many clinical areas, including vascular surgery, cardiology, geriatrics, diabetology, radiology as well as in nephrology. It is a disease of ageing as highlighted by Schwart and White’s oft-quoted post-mortem study from over three decades ago which showed the incidental finding of significant ARVD (defined here as > 50% arterial stenosis) in 42% of patients aged over 75 years (1), irrespective of their cause of death. This degree of prevalence should be of no surprise, as an even greater proportion of aged patients are likely to have significant coronary, cerebrovascular and/or peripheral vascular disease, and ARVD has been shown to be co-morbidly associated with extra-renal vascular by several previous angiographic studies. Hence, 42% of 100 patients investigated for peripheral vascular disease (2) and 15% of 1302 patients who underwent flush aortography at the time of coronary angiography (3) had significant ARVD. A later study on another cohort of patients with ischaemic heart disease from the latter institution, Duke University, showed, as expected, that the likelihood of severe ARVD increased with increasing severity of coronary artery disease (4). Almost 40% of patients with aortic aneurysms will have ARVD, and we have shown that 34% of elderly patients presenting with congestive cardiac failure had significant ARVD (5). There is recent evidence that suggests that the ARVD contributes to the pathogenesis of the heart failure in some of these patients (6).
ARVD and hypertension
Renal artery stenosis (RAS) has been known to predispose to hypertension since Goldblatt’s seminal animal experiments that involved renal arterial clipping. It is now agreed that about 80% of secondary causes of hypertension are due to RAS, which extrapolates to 4% of all hypertensive cases. The pattern is often that of severe systolic hypertension in this ageing population. This may require regimens of multiple drugs for adequate control, and recent randomised-controlled trials have shown the beneficial effects of percutaneous revascularization procedures in hypertension control (as opposed to benefiting renal function) in patients with atherosclerotic renal artery stenosis (7,8).
ARVD and chronic renal failure
There are also countless patients with chronic renal failure (CRF) and ARVD, and the progression of a proportion to end-stage renal failure (ESRF) is delivering a major burden to the renal replacement therapy services of the Western World. A review of the literature over the last two decades would suggest that the prevalence of ESRF due to ARVD has increased almost three-fold (9), but in reality awareness and investigation of the condition has improved, and more elderly patients are now admitted to dialysis programmes. For example, at least 15% of UK dialysis patients will have ARVD, and the prevalence may be greater than 25% in elderly sub-groups. Preventing the progression of renal impairment to ESRF is now a major challenge that confronts the nephrologist who manages patients with ARVD.
Outcomes after renal revascularization in ARVD
Revasularization procedures have been performed in patients with ARVD for many years, often in the belief that they will lead to an improvement or stabilization of renal function. Evidence accumulated within the last few years now challenges the pragmatism of this approach.
Progression of renal artery stenosis (RAS)
The natural history of proximal arterial lesions in ARVD has been defined by previous invasive and non-invasive studies that have demonstrated a rapid rate of progression of high-grade stenoses to renal artery occlusion (RAO) with consequent loss of functioning renal mass. In an early study, 40% of patients with RAS > 75% were shown, with the use of serial angiography, to have progressed to occlusion at two years (10). Serial duplex ultrasound findings have been consistent with this, with 5% of those patients with RAS >60% developing RAO each year (11). It is these studies that have underpinned the rationale to maintain renal arterial patency by angioplasty, with or without endovascular stent placement, or by surgery, in order to preserve renal function in patients with severe RAS. However, more recent investigations, performed in an era when aspirin and lipid-lowering therapy are widely used in patients with ARVD, show a lower rate of RAS progression and that other factors, such as hypertension, may be more important than RAO in determining progression to renal atrophy (12). Analysis of the outcome of patients who undergo revascularization procedures also provide insights into the pathogenesis of renal failure in ARVD patients.
Effects of renal revascularization upon renal function in ARVD
The introduction and refinement of interventional radiological techniques has made renal revascularization available to a broader group of patients. Although many previous reports of outcome after such treatments avail the literature, most are retrospecive reviews of patient series, and the same is true for surgical revascularization. However, critical analysis reveals that the effects of each of the three different revascularization techniques upon renal functional outcome are broadly similar, and they can be summarised as follows. Although an overall improvement in renal function may be seen, sub-group analysis typically shows that the majority of patients (about 50-70%) have no change after intervention, whereas the remaining patients are equally divided (15-25%) into those manifesting progressive deterioration or definite improvement of renal function (13,14). With respect to identification of those patients who are more likely to benefit from revascularization, it appears that patients with only mild renal dysfunction appear to have very good renal functional outcomes. If patency is restored in patients with moderate chronic renal failure (eg serum creatinine 1.5-3.0 mg/ml or 132-265 m mol/l) most studies demonstrate a stabilization of renal dysfunction, rather than an absolute improvement, in the majority of patients undergoing revascularization (13,15). Patients with severe renal failure (creatinine > 3.0 mg/ml or > 265 m mol/l) are more likely to progress to ESRF despite revascularization, but stabilization is still seen in the majority. However, the term ‘stabilization’ needs to be considered cautiously here as many authors actually report that renal function was ‘unchanged’ after revascularization (as opposed to demonstrating that renal function was deteriorating steadily before, and then remained unchanged ie ‘stabilized’, after the procedure).
Use of isotopic techniques to estimate single kidney glomerular filtration rate (SK-GFR) shed further light on the effects of revascularization (16). The SK-GFR can be calculated by using a dual protocol of chromium-labelled EDTA to assess GFR, with DMSA to estimate divided renal function. In patients with ARVD, angioplasty usually did not increase the individual function of the affected kidney (Figure 1), whereas SK-GFR is increased after revascularization of fibromuscular lesions in younger patients, presumably because the renal parenchyma beyond the stenosis is healthy in this condition.
Figure 1
Pathogenesis of renal dysfunction in ARVD
There is no doubt that in some patients with ARVD a drop in renal perfusion, associated with a tight stenosis in the main renal artery, will result in impaired renal function simply due to a hydraulic effect. However, as described above, an improvement in renal function is only recognised in the minority of patients who undergo interventional procedures that restore patency to the renal artery, and it is likely that the renal dysfunction associated with ARVD is multifactorial. There is compelling evidence to support the view that the extent of intra-renal vascular and parenchymal injury, either co-existing with or resulting from proximal ARVD, is the most usual determinant of renal dysfunction in these patients. The results of several recent studies will now be considered.
Renal artery patency and its relationship to renal function
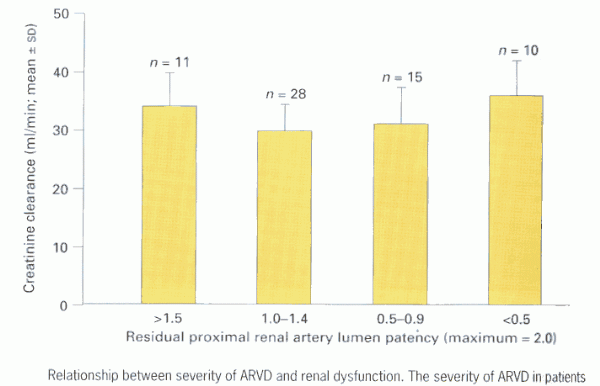
We examined the angiographic findings of a cohort of 71 patients, treated for ARVD at this institution between 1995 and 1997, in the context of their renal function (creatinine clearance) as assessed at the time of imaging (17). The severity of renovascular lesions were arbitrarily represented by residual proximal renal artery lumen patency, which was estimated according to the following scale - bilateral normal = 2.0, unilateral 50% RAS (normal contralateral) = 1.5, unilateral RAO or bilateral 50% RAS = 1.0, RAO with contralateral 70% RAS = 0.3. Patients were stratified into sub-groups based upon their residual patency index. Figure 2 shows that when severity of proximal ARVD and renal dysfunction are examined in this cross-sectional manner, no overall relationship exists between the two; renal dysfunction was equally marked in some patients with mild ARVD as in those with severe bilateral ARVD.
Figure 2
The implications of these findings are supported by studies with SK-GFR (16). Hence, in patients with unilateral RAS, the SK-GFR may be similar or less in that kidney which benefits from the normal renal arterial supply, compared to the kidney supplied by the stenosed artery (Figure 1).
Renal function in ARVD patients with renal artery occlusion (RAO)
As patients with RAO effectively have only a single functioning kidney they constitute an ideal group in whom to study the relationship of ARVD anatomical severity to renal functional outcome. We identified 142 (47.5%) patients with RAO out of 299 ARVD patients who had presented to a single Centre over a 12-year period (18). There was no relationship between baseline renal function and contralateral renovascular anatomy (Figure 3). Patients with contralateral normal, insignificant (< 50%) or significant (> 50%) RAS had baseline creatinine of 243 ± 235, 292 ± 197 or 210 ± 102 m mol/l, respectively, but patients with bilateral RAO (creatinine 540 ± 304 m mol/l; P <0.0001) were significantly worse. However, there were significant correlations between baseline GFR and both proteinuria (r = -0.32, P <0.01) and contralateral bipolar renal length (r = 0.44, P< 0.0001).
Figure 3
There were 85 (59.9%) deaths during follow-up; 9 patients required dialysis at presentation and a further 15 (10.5%) during the course of the study. The median survival of the whole group was 25 months, and 5-year survival 31%. Multivariate analysis demonstrated a clear relationship between low baseline GFR and increased probability of death or dialysis-need, but renal vascular anatomy appeared to have no prognostic impact.
Proteinuria in ARVD patients
Proteinuria has previously been thought to be associated with severe ARVD, and it has been shown to be in the nephrotic range in patients with ‘ischaemically-mediated’ focal segmental
glomerulosclerosis (19). In a study of 92 patients combined from the Guys and Hope Hospital renovascular disease databases, we evaluated the relationship between the degree of proteinuria (24-hour urinary excretion) and renal dysfunction (Figure 4). A clear pattern
emerged, with patients with the worst renal function having the heaviest proteinuria; there was also no relationship between the severity of ARVD and the level of proteinuria (20). Hence, it appears that, provided other pathology such as diabetic nephropathy is excluded, proteinuria can be a useful indicator of the extent of parenchymal injury in ARVD patients having renal dysfunction.
Renal parenchymal injury in ARVD
Renal histological abnormalities may comprise any combination of ischaemic injury (glomerulosclerosis, tubular atrophy and interstitial fibrosis), intra-renal atheroma, hypertensive vascular lesions and cholesterol athero-emboli (Figure 5), such that the overall picture has been termed ‘ischaemic’ or ‘atherosclerotic nephropathy’.
Figure 5
We have recently shown a relationship between these histological abnormalities and renal functional outcome in 25 patients with atherosclerotic nephropathy (21). Histology was graded by a semi-quantitative scoring system (maximum score, indicating severe damage = 12); patients with scores 0-4 had stable renal function (mean change in GFR was +5.3 ml/min/year, the improvement probably resulting from hypertension control) whereas those with severe histological damage (score 9-12) had deteriorating GFRs (-10.1 ml/min/year). Not surprisingly, ARVD patients who show progressive decline of renal function over time have significantly higher baseline levels of proteinuria (22).
Serial duplex ultrasound has been used by Caps et al in order to determine factors which increase progression to renal atrophy in ARVD (12). Their findings, that the cumulative risk of atrophy was greatest in patients with poorly controlled hypertension, and that atrophy of kidneys with RAS > 60% was usually not due to development of RAO, are consistent with our current views, which, to re-iterate, are that the extent of renal parenchymal damage is usually the major arbiter of renal functional outcome in patients with ARVD.
Prognosis of patients with ARVD
The high prevalence of extra-renal vascular disease underlies the very high relative mortality risk of patients with ARVD. Previous studies have indicated that the severity of the proximal renal arterial lesions appears to be a marker of the severity of vascular disease elsewhere in the patient, and mortality is, therefore, can be directly related to initial angiographic findings. For example, the two year survival of patients with unilateral RAS or RAO is more than 90% which contrasts with a figure of less than 50% for patients with severe bilateral ARVD (23). There is also a strong link between decreasing renal function and mortality, and this was clear in our study of patients with RAO (18), described above. Survival characteristics of all ARVD patients on our database also highlight the importance of extra-renal vascular disease in determining prognosis. Hence, those patients who presented with normal renal function, or ARVD without other vascular disease, had 5-year survivals of 78.3% and 80.1%, respectively, compared to 34.8% (severe renal failure) and 36.4% (patients also having both peripheral vascular and ischaemic heart disease) survival in patients with more advanced disease. Once ARVD patients reach the dialysis programmes they have a very poor outlook, with even a higher likelihood of mortality than diabetics; mean survival on dialysis may only be 27 months and 5-year survival 18% (9).
In summary, patients with ARVD have a greatly increased risk of mortality if they have moderate-severe renal failure at presentation. Intra-renal damage within the kidneys appears to be a major contributor to the pathogenesis of the renal failure in these patients, and this is especially true of the contralateral ‘normal’ kidney in many patients with unilateral RAS or RAO. By inference, future therapeutic strategies in these high-risk patients should be aimed at minimising intra-renal damage before renal failure supervenes. Large-scale trials are also now warranted in ARVD patients, with the hope that those sub-groups most likely to gain renal functional and mortality benefits from revascularization procedures can be identified.
REFERENCES