
Roland Pilgram and Daniel Schneditz
| DISCUSSION BOARD |
Introduction
It is well accepted that the treatment of end stage renal disease patients (ESDR) has to be tailored and personalized to individual needs. Adaptive control has the potential to replace the manual adjustment of main treatment variables such as ultrafiltration rate (UFR), dialysate temperature (TD), and dialysate conductivity (DC). The management of these variables is becoming increasingly difficult because of the complex relationship between the primary perturbations caused by the treatment and the response from the body which requires continuous adjustments instead of a constant and predetermined setting. Therefore, it is the ultimate goal to provide automatic control in hemodialysis. Furthermore, the treatment should improve patient comfort and be carried out without use of additional body sensors and without additional medication. Automatic control of hemodialysis has the potential to provide a better treatment to the ever increasing number of ESDR patients who present with more complicated co-morbid conditions.
Control Systems
Controller and controlled system (plant) are the essential constituents of any control system, together with actuators and sensors. Depending on their connection two distinct configurations can be described [1].
Forward Control System
The forward control system operates on the plant using actuators independently of the actual output (Figure 1). A priori knowledge of the plant characteristics and of the disturbances is mandatory otherwise the forward approach results in a poor precision of the controlled output.
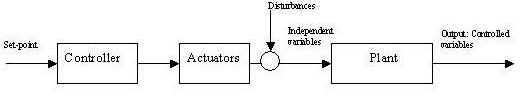
Figure 1. Forward control system
The current dialysis treatment is managed in a typical forward manner, for example in the prescription and delivery of the dose of dialysis measured as Kt/V [2] [3]. The medical staff acts as a part of the controller and determines time on dialysis (t) and clearance (K) for the estimated patient volume (V) to reach the prescribed Kt/V (set point). Disturbances such as errors in actual blood flow because of erroneous pump calibration, changes in dialyzer clearance because of fiber clotting, and reduction in effective clearance because of recirculation and compartment effects lead to a difference between the delivered (controlled variable) and the prescribed dose (set point) of dialysis [2] [4]. In the forward control system there is no means to compensate for this difference because there is no information on the actual output.
Feedback Control System
The control of the system output is improved when the controller receives information from the actual output in the so-called feedback control system. In the example to control dose of dialysis effective clearance must be measured and compared to prescribed clearance [5]. From the difference between delivered and prescribed clearance (negative feedback) the controller adjusts different actuators such as blood flow, dialysate flow, and treatment time to compensate for the error between output and set-point. (Figure 2). In this case the controller does not require a complete and detailed knowledge of the plant.
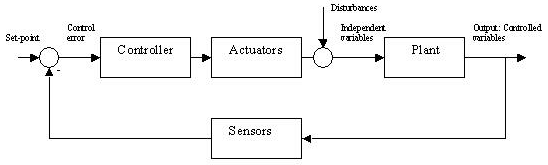
Figure 2. Feedback control system
The information of the error signal for designing a control system can be used in different ways. Controllers may operate in proportion to the magnitude of the error (P), they may use the time derivative of the error (D), or the integral error accumulated over a period of time (I) to construct the signal used to drive the actuators. A controller that combines all three terms, the so-called proportional-integral-derivative (PID) controller can significant improve response times, damping, and steady-state error.
The machine and Patient system
The machine receives information from the medical staff regarding the UF volume (UFV) together with a specific UF profile and the treatment time (t) derived from the dose of dialysis (Kt/V) which depends on patient volume (V) and dialyzer clearance (KD) determined by the dialyzer mass transfer coefficient (K0A), the dialysate flow (QD) and the effective blood flow (QB). Blood electrolytes, most importantly the concentration K+, Na+ and HCO3-determine the composition of the dialysate bath.
If the automatic administration of pharmacological compounds is excluded the actions of the machine (actuator) on the plant (patient) are limited to fluid removal (UFR), blood flow (QB), dialysate flow (QD), dialysate temperature (TD), and dialysate composition, essentially dialysate sodium [Na+]D, and dialyzer clearance (KD) (Figure 3).

Figure 3. Feedback control of hemodialysis. The set point for system output is determined by dose of dialysis (Kt/V), prescribed weight loss (UFV), and electrolyte status (ES). Machine inputs are marked in green, machine outputs (actuators) are marked in blue, and patients outputs are marked in red. Feedback from the patient to the machine is divided into two parts: variables derived from the extracorporeal circulation and the blood, and patient variables describing the physical condition of the patient. (Abbreviations: UFV: ultrafiltration volume, t: treatment time, ES: electrolyte status, QB: blood flow, QD: dialysate flow, KD: dialyzer clearance, UFR: ultrafiltration rate, TD: dialysate temperature, [Na+]D: sodium concentration of the dialysate, RBV: relative blood volume, TB: blood temperature, Keff: effective body clearance, HR: heart rate, BP: blood pressure)
As can be seen in Figure 3 the dialysis machine is an extracorporeal component of the cardiovascular system and interacts with it. The main actuator with direct influence on blood volume and indirect influence to the hemodynamic stability of the cardiovascular system is the UFR.
Blood Volume Changes and control
Ultrafiltration induced hypovolemia is one of the main causes for intradialytic morbid events [6]. with the blood volume during hemodialysis depending on ultrafiltration (UF) and vascular refilling. If vascular refilling rate does not match UF rate, blood volume will drop during the treatment [7] [8]. Failure to compensate for a critically low blood volume eventually leads to symptomatic hypotension, which requires immediate attention. Since fluid removal by ultrafiltration is accompanied by a decrease in blood volume, one of the current approaches to reduce intradialytic morbid events is to avoid excessive blood volume reductions. This problem requires to define the ideal blood volume trajectory from the overhydrated state to the state at target weight.
Tracking Problem
A trajectory describes the path taken by a variable such as blood volume from the starting point to the target. The multi-compartment nature of body fluid distribution predicts that at a constant UF-rate blood volume at the end of the treatment will drop below the desired equilibrium (Figure 4, left panel). Blood volume will rebound to the desired equilibrium after the end of ultrafiltration. The drop of blood volume below the target equilibrium could be considered as excessive and could be prevented by an optimized UF-rate strategy. One would assume that a linear blood volume trajectory from overhydration to the target weight represents an ideal trajectory (Figure 4, left panel). It can be shown that such a trajectory is impossible for theoretical and practical reasons.
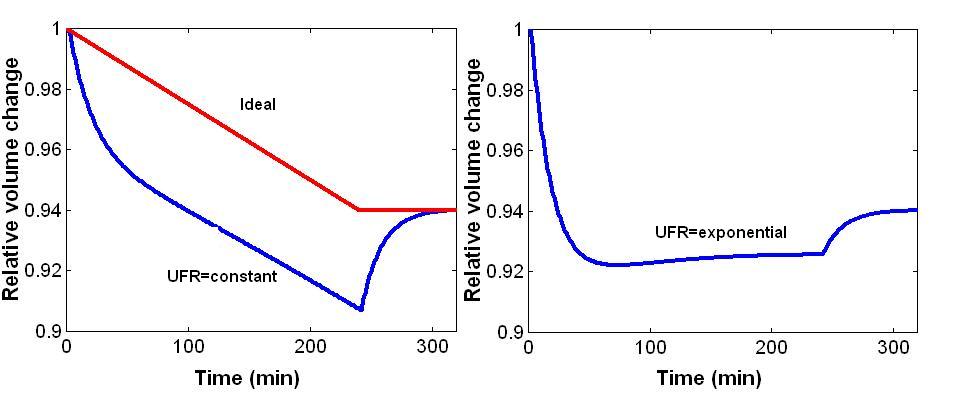
Figure 4. Volume trajectories. Left panel: Ideal volume change (red line) and expected volume change with constant UFR producing an excessive drop in blood volume and a rebound after the end of ultrafiltration (blue line). Right panel: Volume change with exponential decline in UFR with high UFR at the beginning and low UFR at the end of the ultrafiltration phase (notice the reduction in blood volume rebound).
However, it can be shown that an increase in UF-rate above the value for constant ultrafiltration early during dialysis and a reduction of UFR throughout the treatment will enhance vascular refilling early during hemodialysis and reduce the excessive drop of blood volume late during dialysis (Figure 4, right panel). This appears to be a promising approach since the risk for intradialytic morbid events increases in the second half of the treatment session. The optimized fluid removal profile may be used either in the forward control system or in the feedback control system as the set point profile for the controller.
The complexity of the hemodialysis
Cardiovascular stability also depends on other factors such as body temperature which is influenced by dialysate temperature (TD). On the one hand cooling of body temperature by dialysate activates mechanisms for increasing the total peripheral resistance supplying cardiovascular stability [9] [10], on the other hand fluid removal is combined with heat retention causing the opposite effect [11]. A more detailed analysis of this problem is presented in a companion paper of this conference.
The electrolyte composition of dialysis fluid may also influence cardiovascular stability. Lowered sodium concentration [Na+]D reduces vascular refilling and contributes to hypovolemia [12] [13]. Thus different outputs from the machine (UFR, TD, [Na+]D) have different contributions to the physical condition of the patient. Nearly every patient output depends on every input in a complex way. In terms of control theory this structure refers to a multiple input multiple output system (MIMO).
Despite the high technological level reached in the dialysis machine, outputs provided from the patient by extracorporeal sensors and typical patient data such as heart rate (HR) and blood pressure (BP) are not integrated in standard feedback systems. Which patient information may be best suited for a feedback control system?
Proposed feedback systems
Several variables may be used in feedback control to automate dialysis therapy (Figure 3). New current systems are already dealing with feedback control mechanism controlling either the dialysate temperature (TD) or fluid removal (UFR), or with the feedback control of two actuators such as sodium concentration [Na+]D and fluid removal (UFR). Apart from simple on-off techniques to more complex fuzzy logic controllers [14] only two systems are commercially available today, the Hospal and the Fresenius system.
The Hospal System
The Hospal system is designed to control the trajectory of relative blood volume changes by adjusting the dialysate composition and the UF rate of the hemodialysis and UF process [15]. The control system requires the definition of a target for the three variables incorporated into this control, i.e. weight reduction, dialysate conductivity, and blood volume change. The rationale for inclusion of dialysate conductivity is based on the effects of sodium concentration on extracellular volume, vascular refilling, and blood volume preservation [12] [16][16]. Thus, in this system vascular refilling early during dialysis is enhanced both by high UF rate and by high dialysate sodium concentration. Based on the target values for weight change and treatment duration the system determines a trajectory for intradialytic blood volume changes, which are to be followed with a certain tolerance. UF rate is limited by a maximum rate of 2 L/h. Dialysate conductivity is controlled by the goal to provide the same sodium mass balance as a comparable treatment with constant dialysate conductivity. Calculations are based on the conductivity of the dialysate bath and on a sodium kinetic model. The control algorithm does not have access to a direct on-line measurement of plasma sodium concentration so that there is no feedback control of this system variable. Upper and lower bounds of 13.5 and 16 mS/cm limit dialysate conductivities, respectively. Failure to remain within the bounded region leads to specific alarms informing the operator when the control goal cannot be achieved. The system utilizes exponential blood volume trajectories derived from considerations outlined above. UF profiles obtained with this control system are anything but smooth. However, there is a trend for declining UF rates as expected for exponential blood volume trajectories. UF rates are higher than average at the beginning of the treatment and gradually decrease towards the end of the treatment (Figure 5).
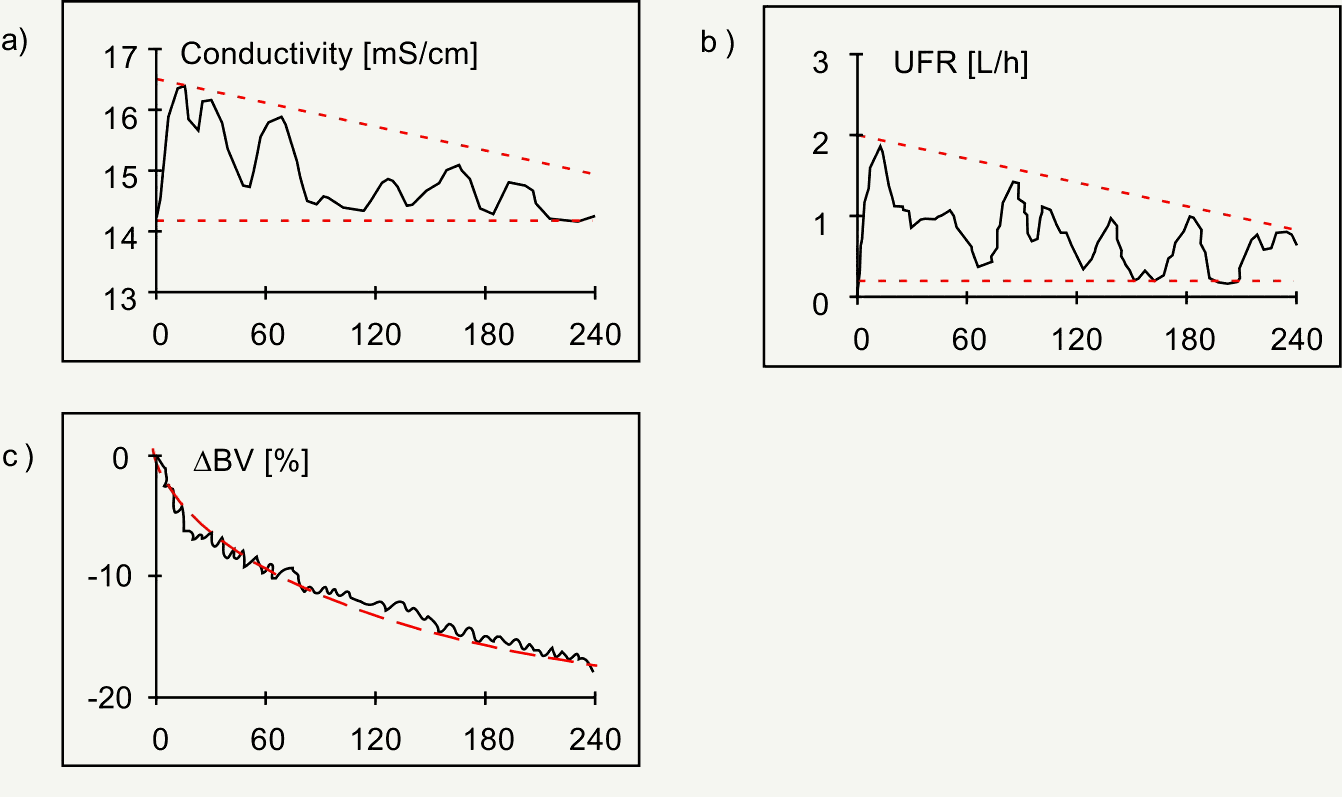
Figure 5. Hospal Hemocontrol. Continuous adjustment of conductivity (panel a) and ultrafiltration rate (UFR, panel b), both within given limits (broken lines), to follow the predetermined trajectory of relative blood volume changes (D BV, broken line, panel c). Note that both conductivity and UFR is high at the beginning of the treatment to maximize vascular refilling early during dialysis.(courtesy of Hospal, Lion, France).
The Fresenius System
The control system is based on the definition of a critical current blood volume and on declining UF rates [17]. However, the system does not track a pre-defined blood volume trajectory and does not aim to reach a target blood volume reduction. Controlled fluid removal is achieved by a continuous determination of UF rates according to the following rules: a) volume must be removed within the treatment time; b) initial UF rate is set at twice the constant UF rate; c) if RBV drops more than half of the distance between the current RBV and the critical RBV, UF rate is linearly decreased. Thus, in a treatment without a drop of RBV below half of the distance between the current and critical RBV, the algorithm provides a linear decrease in UF rate, with UF starting at twice the constant UF rate and reaching zero UF rate at the end of the treatment. However, when RBV falls below half of the distance between the current and critical RBV, UF rate is further reduced such as defined by rule b) with the consequence that UF at the end of the treatment can no longer be zero (Figure 6).
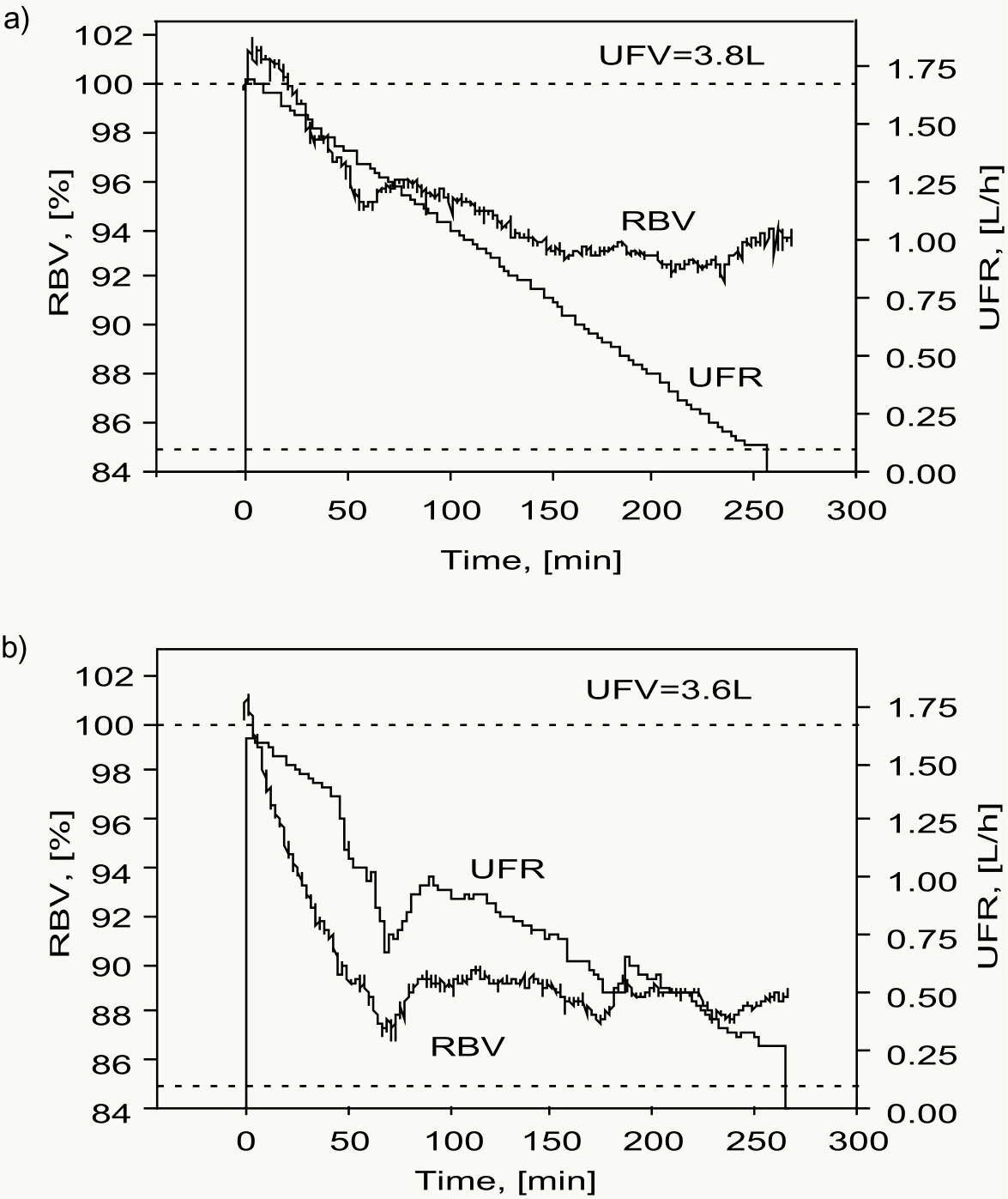
Figure 6. Two consecutive dialysis treatments in the same patient using feedback control of relative blood volume RBV. Two consecutive dialysis treatments in the same patient using feedback control of relative blood volume (RBV) and a critical RBV of 85%. Initial ultrafiltration rate (UFR) was doubled and subsequently decreased as required by the control algorithm. In the treatment shown in the top panel (a) RBV remained well above the critical RBV and UFR was linearly reduced throughout the treatment phase. In the treatment shown in the bottom panel (b) RBV fell below half of the distance of the current to the critical RBV at about t=50 min and UFR was reduced according to the control algorithm. RBV recovered at t=90 min and UFR was transiently increased for the next hour (reprinted with permission of Blackwell Science, Inc. and the editor of Seminars in [17]).
However, the system does not include a control of dialysate conductivity, but it can operate in combination with the temperature-control system offered by a blood temperature monitor.
Even though both systems are grounded on measuring the same system output, i.e., relative blood volume changes, they are based on different approaches. But a combined control of the three main aspects of hemodialysis as control of fluid removal, control of dialysate temperature, dialysate composition, has not yet been realized.
Clinical Studies
The first prospective study conducted by Santoro et al. done for two periods of one month in eight hypotensive patients showed that the pre- to postdialysis arterial blood pressure changes, the number of severe hypotensive episodes, the overall incidence of complaints, especially of muscular cramps, and the need for therapeutically administered saline in each session were significantly reduced in controlled treatments using the Hospal system [15]. Using the same system in 12 hypotension prone patients in a short-term prospective cross-over design Ronco et al. observed a significant reduction in the occurrence and severity of hypotensive episodes, respectively, as well as an increase in equilibrated Kt/V in treatments performed with automatic feedback control of relative blood volume changes [18]. The results show that cardiovascular stability also provides a positive effect on urea kinetics most likely because of an adequate blood flow distribution to all organ systems. In the most recent study conducted by Basile et al. using the same system in 19 hypotension prone patients for a duration of 14 to 30 months overall occurrence of symptomatic hypotension and muscle cramps decreased [19].
Open Problems and Conclusion
Even though the clinical studies using feedback control of relative blood volume show an impressive reduction in intradialytic morbid events, one would expect optimum control to prevent all treatment complications. Whether this is related to uncertainties of the measured output variables such as relative blood volume, to the missing control of other variables, to the constraints determined by the prescription of targets to be reached within a given time, or whether this is related to other conceptual shortcomings remains to be recognized in future.
Could it be that relative blood volume is not the relevant variable to be controlled during UF? Current techniques assume that blood volume behaves like a single compartment, arterial, venous, and microcirculatory volumes together. Thus, a measured decline in blood volume cannot indicate which of these volumes really decreases. However, to defend against a decline in blood pressure and to assure diastolic filling, it is important to maintain a high central venous pressure, probably by means of a high central blood volume.
The systems discussed in this chapter control for one or two patient variables at their best. However, for the full benefit the feedback control system would have to integrate all aspects of a controlled perturbation such as UFR, TD, [Na+]D, QD and KD. But even such a system will be far from exhibiting the full benefits of feedback control. Take UFR as an example. Current systems still require an input regarding the UF volume, the relative blood volume limit or the desired target volume reduction. However, the ultimate goal of UF is to normalize blood pressure and the intrinsic blood pressure control system with the least rate of complications in the shortest time possible. Thus, an integrated feedback system must be able to autonomically prescribe and remove fluid depending on the degree of overhydration and under the constraints of hemodynamic stability, also with regard to the time dependence of internal parameters and their variations, such as aging, temperature and the physical condition.
Acknowledgement
This work was supported by the Austrian Science Foundation, project F323.
References:
2. Depner TA: Assessing adequacy of hemodialysis: urea modeling. Kidney Int 45:1522-1535, 1994 3. Gotch FA: Kt/V is the best dialysis dose parameter. Blood Purif 18:276-285, 2000 4. Schneditz D, Daugirdas JT: Compartment effects in hemodialysis. Sem Dial 14:271-277, 2001 5. Lindsay RM, Sternby J: Future directions in dialysis quantification. Semin Dial 14:300-307, 2001 6. Kaufman AM, Krämer M, Godmere RO, Morris AT, Amerling R, Polaschegg HD, Levin NW: Hemodialysis access recirculation (R) measurement by blood temperature monitoring (BTM) - A new technique. (abstract) J Am Soc Nephrol 2:324, 1991 7. Bonnie E, Lee WG, Stiller S, Mann H: Influence of fluid overload on vascular refilling rate in hemodialysis: Continuous measurements with conductivity method, in Progress in Artificial Organs, edited by Nose Y, Kjellstrand CM, Ivanovich P, Cleveland, ISAO Press, 1986, pp 135-137 8. Schneditz D, Roob JM, Oswald M, Pogglitsch H, Moser M, Kenner T: Nature and rate of vascular refilling during hemodialysis and ultrafiltration. Kidney Int 42:1425-1433, 1992 9. Mahida BH, Dumler F, Zasuwa G, Fleig G, Levin NW: Effect of cooled dialysate on serum catecholamines and blood pressure stability. ASAIO Transactions 29:384-389, 1983 10. Maggiore Q, Pizzarelli F, Sisca S, Catalano C, Delfino D: Vascular stability and heat in dialysis patients. Contrib Nephrol 41:398-402, 1984 11. Rosales LM, Schneditz D, Morris AT, Rahmati S, Levin NW: Isothermic hemodialysis and ultrafiltration. Am J Kidney Dis 36:353-361, 2000 12. Fleming SJ, Wilkinson JS, Greenwood RN, Aldridge C, Baker LR: Effect of dialysate composition on intercompartmental fluid shift. Kidney Int 32:267-273, 1987 13. de Vries JPPM, Bogaard HJ, Kouw PM, Oe LP, Stevens P, de Vries PMJM: The adjustment of post dialytic dry weight based on non-invasive measurement of extracellular fluid and blood volumes. ASAIO J 39:M368-M372, 1993 14. Schmidt R, Roeher O, Hickstein H, Korth S: Prevention of haemodialysis-induced hypotension by biofeedback control of ultrafiltration and infusion. Nephrol Dial Transplant 16:595-603, 2001 15. Santoro A, Mancini E, Paolini F, Cavicchioli G, Bosetto A, Zucchelli P: Blood volume regulation during hemodialysis. Am J Kidney Dis 32:739-748, 1998 16. Mann H, Stefanidis I, Reinhardt B, Stiller S: Prevention of haemodynamic risk by continuous blood volume measurement and control. Nephrol Dial Transplant 11:S48-S51, 1996 17. Krämer M: New strategies for reducing intradialytic symptoms. Sem Dial 12:389-395, 1999 18. Ronco C, Brendolan A, Milan M, Rodeghiero MP, Zanella M, La Greca G: Impact of biofeedback-induced cardiovascular stability on hemodialysis tolerance and efficiency. Kidney Int 58:800-808, 2000 19. Basile C, Giordano R, Vernaglione L, Montanaro A, De Maio P, De Padova F, Marangi AL, Di Marco L, Santese D, Semeraro A, Ligorio VA: Efficacy and safety of haemodialysis treatment with the Hemocontrol biofeedback system: a prospective medium-term study. Nephrol Dial Transplant 16:328-334, 2001