
|
Paneles de Discussión
Paneais de Discussio |
"CHRONIC ALLOGRAFT DYSFUNCTION: ARE WE ABLE TO DISCRIMINATE CHRONIC REJECTION FROM CHRONIC CNI NEPHROPATHY?"Rene C Bakker,Leiden University Medical Center. Leiden, The Netherlandsrnr@euronet.nlIntroduction The subject of chronic rejection of allografted kidneys has received increasing attention in recent years and newer morphometric tools have been developed to demonstrate this condition. The electron microscopic evaluation of the basement membrane of the peritubular capillaries is most likely a valuable new tool. The significance of a positive staining for C4d is not fully clear. These newer tests may suggest the presence of chronic rejection but do not exclude a co-existing toxic influence of a calcineurin inhibitor. The impact of chronic cyclosporine nephrotoxicity The specific understanding of the importance of chronic CsA nephrotoxicity (CsAT) in renal transplantation has long been hampered by the lack of specific and sensitive markers of this condition and the absence of studies with long term follow-up. CsAT may affect the allografted kidney rather slowly and it may take many years before the real impact is evident in clinical trials. Data derived from studies on patients with various autoimmune disease or solid organ transplants other than a kidney treated with CsA have indicated a relative high incidence of chronic CsAT 1-11. In heart transplant patient’s end-stage renal failure has been observed in 6-10%, the frequency of which apparently increases with a longer period of follow-up 12. Heart transplant patients receive relatively high doses of CsA because of fear of rejection. However, renal functional or structural changes have also been observed frequently in patients on lower doses of CsA treated for autoimmune disease. Two studies examined patients with psoriasis and included pre and post treatment protocol biopsies 8;11. Biopsies taken at 1 year showed de novo interstitial fibrosis in more than 40% of patients in the study of Svarstad 8. Zachariae reported a histological follow-up of 25 patients 11. Seventeen patients had normal baseline histology and at two years all had histological changes compatible with chronic CsAT. At four years all studied biopsies (n=11) displayed moderate to severe fibrosis. More direct evidence of the importance of chronic CsAT after allograft transplantation has come from recently analyzed data from two azathioprine conversion studies with 15 years of follow-up 13, and two studies that evaluated the conversion to MMF in patients with established aspecific chronic allograft nephropathy (CAN) 14;15. The fifteen year data from our open-label prospective randomized study that compared cyclosporine continuation with conversion to azathioprine three months after transplantation showed a higher risk of CAN in the group that continued cyclosporine (relative risk 4.3, 95% CI: 1.4 to 12.9) 16;17. We studied predominantly Caucasian recipients, generally well matched for HLA antigens. A better death censored graft survival was observed in the azathioprine group after two years post-transplantation The 15 years death censored graft survival was 81.9 vs. 69.2 % (p=0.012). These results are in line with data from a not yet published multicenter Australian conversion study with an identical follow-up. In this study a highly significant difference in mean graft survival was found favoring CsA usage shorter than 6 months (13.3 vs. 11.8 years, p <0.01)13. Sparing of cyclosporine in patients with established CAN has been studied with MMF as the added agent. 14. The slope in GFR decline pre- and post-intervention was compared. Fifty to sixty percent of the patients treated with either reduced dose of CsA (n=67) or tacrolimus (n=33) showed an improvement in the rate of decline and more than 90% of the patients that were withdrawn from CNI therapy (n=18). A small risk of AR was noted. A second ongoing multicenter study examined the withdrawal of CsA in patients with gradually declining allograft function. After 6 months stabilization or improvement was seen more often (58% vs. 32%) in the patients that converted to MMF (n=73) as compared to the ones that continued on CsA (N=70)15. The pathologic diagnosis of chronic CsA nephrotoxicity The lesions that are most frequently found in native kidneys with chronic CsAT are non-specific and include: tubular atrophy, interstitial fibrosis, slight mononuclear cell infiltration, Bowman’s capsule basement membrane thickening, glomerular collapse, global sclerosis and non-specific arteriolar hyalinosis as seen in hypertension or diabetes. Glomerular thrombosis or necrosis and signs of arteriolar thrombotic microangiopathy are very rarely observed with the use of lower doses of CsA 18, 19. Also tubular alterations including honeycomb vacuolization of the proximal tubular epithelium, giant mitochondria and micro-calcifications or minor changes of endothelial or smooth muscle cells occur infrequently 19. The most specific marker of CsAT is a nodular hyaline insudation in the periphery of small arterioles either patchy or circumferential designated as peripheral nodular hyaline degeneration (PNHD) 19-21. Arterioles up to two layers of smooth muscle cells are involved. Electron microscopy have suggested that the deposits of PNHD occur at sites of myocyte necrosis 19;22;23. Immunofluorescence staining is often positive for IgM and C3 19, representing aspecific binding of plasma proteins. PNHD must be differentiated from the arteriolar hyaline changes as seen in diabetes or long-standing hypertension, where the hyaline insudation occurs on the inside of the SMC layer 19. The differentiation may be difficult on light microscopy, especially when the lesions are more pronounced. PNHD may regress or even completely vanish upon reduction of dose or withdrawal of CsA 24-27. The hallmark of irreversible chronic CsAT is the occurrence of tubulointerstitial and glomerular changes including segmental and global glomerulosclerosis, interstitial fibrosis and tubular atrophy 11;19;22;28-30. The tubulointerstitial changes may be found before renal function is impaired 4, and in a rat model even before PNHD occurs 31. The severity of the tubulointerstitial changes correlates well with the degree of glomerular sclerosis 22. Non affected glomeruli are hypertrophied 32-34, and presumably preserve function by hyperfiltration. In a series of 192 patients treated with CsA for various autoimmune diseases 26% of the biopsies showed interstitial fibrosis, however PNHD was only noted in 4% 35. Aspecific arteriolar hyalinosis was observed more frequently. In another study with protocol biopsies of uveitis patients a high incidence of arteriolar hyaline changes was found that increased steadily with time 11. However, in this study it was not clear if the specific PNHD lesion was scored. In 1994, an international advisory board of nephropathologists with extensive experience in the evaluation of kidney biopsies of patients on CsA, found that the reproducibility and diagnostic reliability of the evaluation of arteriolar lesions including PNHD was poor 35. In contrast, the interobserver variation on tubulointerstitial changes was low. The diagnosis of chronic CsAT in allografted kidneys is therefore difficult. The nature of changes in the vascular and glomerular compartments may sometimes be suggestive of an etiologic factor in graft deterioration but do exclude concommitant chronic CsAT. Allograft glomerulopathy with reduplication of the GBM indicates an allodependent insult at the level the glomeruli and is found in a minority of late biopsies 36-41. Concentric intimal thickening of arteries and arterioles may result from chronic rejection but is less specific 40;42;43. A high degree of multilayering of the peritubular capillary basement membrane of is thought to indicate chronic rejection. When PNHD is found CsAT should be considered. Striped fibrosis in renal allografts is non specific 44. It is clear that the pathologic diagnosis of chronic CsAT in allografted kidneys still needs further improvement by finding a more specific and sensitive marker. Newer tools in studying the cause of chronic allograft nephropathy Because the tubulointerstitial compartment is most frequently involved in chronic CsAT we decided to study the interstitial matrix and searched for specific markers at the molecular level. We studied biopsy samples from two well defined groups of patients suffering from either chronic allograft rejection or chronic CsAT. Various matrix molecules were evaluated by computerized immunohistochemistry and also mRNA expression was studied by RT-PCR . In the immunohistochemistry study we examined the proteins collagen I, III, and IV, collagen IV Biopsy samples were studied by routine light microscopy and after immunostaining. The mean interstitial fibrosis score was significantly higher of the CR and CAN group than of the chronic CsAT group. The cortical tubulointerstitial areas of the CR or CAN groups, but not of the chronic CsAT group, contained more collagen I than normal controls. A difference was already noted in biopsies with mild fibrosis. Collagens III, IV and IV In the mRNA study we examined mRNA levels of transforming growth factor What do these two studies tell us ? Do we have new tools to evaluate the presence of CR or chronic CsAT? We think that the results of the first study showed that indeed differences could be found in the composition of the tubulointerstitial matrix related the cause of allograft deterioration. The study also refuted data from an earlier study that claimed that positive staining for collagen IV In the mRNA study however we did find in the laminin We conclude that the pathologic recognition of chronic CsA nephrotoxicity in allografted kidneys is still imperfect and needs further improvement. Differences in the composition of the tubulointerstitial matrix can be found in kidney allografts that suffer from either CR or chronic CsAT. An increase in laminin 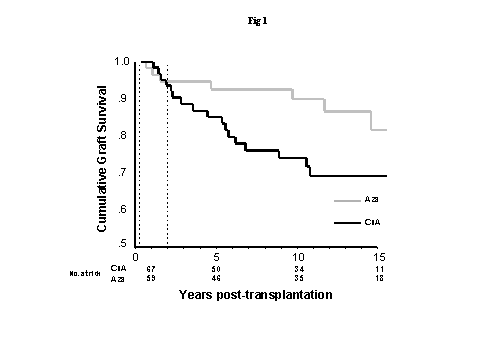 Figure 1. Graft survival censored for death with a functioning graft of the patients that stayed on the drug they were initially assigned to. The vertical dashed lines indicate the start of the study three months post-transplantation (left), and the start of the period of survival benefit in the azathioprine arm of the study two years post-transplantation (right). 16
1. Andersen CB, Larsen S, Elmgreen J, Tvede N, Andersen V: Cyclosporine-induced renal morphologic and immunohistologic changes in patients with chronic uveitis. APMIS 99:576-582, 1991 2. Austin HA, III, Palestine AG, Sabnis SG, Balow JE, Preuss HG, Nussenblatt RB, Antonovych TT: Evolution of ciclosporin nephrotoxicity in patients treated for autoimmune uveitis. Am J Nephrol 9:392-402, 1989 3. Feutren G, Mihatsch MJ: Risk factors for cyclosporine-induced nephropathy in patients with autoimmune diseases. International Kidney Biopsy Registry of Cyclosporine in Autoimmune Diseases. N Engl J Med 326:1654-1660, 1992 4. Fioretto P, Steffes MW, Mihatsch MJ, Strom EH, Sutherland DE, Mauer M: Cyclosporine associated lesions in native kidneys of diabetic pancreas transplant recipients. Kidney Int 48:489-495, 1995 5. Lowe NJ, Wieder JM, Rosenbach A, Johnson K, Kunkel R, Bainbridge C, Bourget T, Dimov I, Simpson K, Glass E, Grabie MT: Long-term low-dose cyclosporine therapy for severe psoriasis: effects on renal function and structure. J Am Acad Dermatol 35:710-719, 1996 6. Messana JM, Johnson KJ, Mihatsch MJ: Renal structure and function effects after low dose cyclosporine in psoriasis patients: a preliminary report. Clin Nephrol 43:150-153, 1995 7. Powles AV, Hardman CM, Porter WM, Cook T, Hulme B, Fry L: Renal function after 10 years' treatment with cyclosporin for psoriasis. Br J Dermatol 138:443-449, 1998 8. Svarstad E, Helland S, Morken T, Bostad L, Myking A, Iversen BM, Ofstad J: Renal effects of maintenance low-dose cyclosporin A treatment in psoriasis. Nephrol Dial Transplant 9:1462-1467, 1994 9. Vercauteren SB, Bosmans JL, Elseviers MM, Verpooten GA, De Broe ME: A meta-analysis and morphological review of cyclosporine-induced nephrotoxicity in auto-immune diseases. Kidney Int 54:536-545, 1998 10. Young EW, Ellis CN, Messana JM, Johnson KJ, Leichtman AB, Mihatsch MJ, Hamilton TA, Groisser DS, Fradin MS, Voorhees JJ: A prospective study of renal structure and function in psoriasis patients treated with cyclosporin. Kidney Int 46:1216-1222, 1994 11. Zachariae H, Kragballe K, Hansen HE, Marcussen N, Olsen S: Renal biopsy findings in long-term cyclosporin treatment of psoriasis. Br J Dermatol 136:531-535, 1997 12. Burdmann EA, Yu L, Andoh TF, Perico N, Bennett WM: Calcineurin inhibitors and sirolimus, in De Broe ME, Porter GA, Bennett WM, Verpooten GA (eds): Clinical Nephrotoxins, chap 21. 2003, 2003, pp 403-458 13. Gallagher, M. P., Eris , J. M., Tiller, D. J., and Hall, B. M. Long term outcome of australian multicenter trial of cyclosporin withdrawal in cadaveric renal transplantation. 26-8-2003. 14. Weir MR, Ward MT, Blahut SA, Klassen DK, Cangro CB, Bartlett ST, Fink JC: Long-term impact of discontinued or reduced calcineurin inhibitor in patients with chronic allograft nephropathy. Kidney Int 59:1567-1573, 2001 15. Dudley C: MMF substitution for CsA is an effective and safe treatment of chronic allograft dysfunction: results of a multi-center randomized controlled study. Am J Transplant 2 (suppl 3):148, 2002 16. Bakker RC, Hollander AA, Mallat MJ, Bruijn JA, Paul LC, de Fijter JW: Conversion from cyclosporine to azathioprine at three months reduces the incidence of chronic allograft nephropathy. Kidney Int 64:1027-1034, 2003 17. Meier-Kriesche HU, Kaplan B: Cyclosporine microemulsion and tacrolimus are associated with decreased chronic allograft failure and improved long-term graft survival as compared with sandimmune. Am J Transplant 2:100-104, 2002 18. Neild GH, Reuben R, Hartley RB, Cameron JS: Glomerular thrombi in renal allografts associated with cyclosporin treatment. J Clin Pathol 38:253-258, 1985 19. Mihatsch MJ, Thiel G, Ryffel B: Histopathology of cyclosporine nephrotoxicity. Transplant Proc 20:Suppl 3):759-771, 1988 20. Mihatsch MJ, Ryffel B, Gudat F: Morphological criteria of chronic rejection: differential diagnosis, including cyclosporine nephropathy. Transplant Proc 25:2031-2037, 1993 21. Mihatsch MJ, Morozumi K, Strom EH, Ryffel B, Gudat F, Thiel G: Renal transplant morphology after long-term therapy with cyclosporine. Transplant Proc 27:39-42, 1995 22. Griffiths MH, Crowe AV, Papadaki L, Banner NR, Yacoub MH, Thompson FD, Neild GH: Cyclosporin nephrotoxicity in heart and lung transplant patients. QJM 89:751-763, 1996 23. Yamaguchi Y, Teraoka S, Yagisawa T, Takahashi K, Toma H, Ota K: Ultrastructural study of cyclosporine-associated arteriolopathy in renal allografts. Transplant Proc 21:1517-1522, 1989 24. Versluis DJ, Ten Kate FJ, Wenting GJ, Jeekel J, Weimar W: Histological lesions associated with cyclosporin: incidence and reversibility in one year old kidney transplants. J Clin Pathol 41:498-503, 1988 25. Morozumi K, Thiel G, Albert FW, Banfi G, Gudat F, Mihatsch MJ: Studies on morphological outcome of cyclosporine-associated arteriolopathy after discontinuation of cyclosporine in renal allografts. Clin Nephrol 38:1-8, 1992 26. Franceschini N, Alpers CE, Bennett WM, Andoh TF: Cyclosporine arteriolopathy: effects of drug withdrawal. Am J Kidney Dis 32:247-253, 1998 27. Hamahira K, Iijima K, Tanaka R, Nakamura H, Yoshikawa N: Recovery from cyclosporine-associated arteriolopathy in childhood nephrotic syndrome. Pediatr Nephrol 16:723-727, 2001 28. Palestine AG, Austin HA, Balow JE, Antonovych TT, Sabnis SG, Preuss HG, Nussenblatt RB: Renal histopathologic alterations in patients treated with cyclosporine for uveitis. N Engl J Med 314:1293-1298, 1986 29. Myers BD, Ross J, Newton L, Luetscher J, Perlroth M: Cyclosporine-associated chronic nephropathy. N Engl J Med 311:699-705, 1984 30. Myers BD, Newton L, Boshkos C, Macoviak JA, Frist WH, Derby GC, Perlroth MG, Sibley RK: Chronic injury of human renal microvessels with low-dose cyclosporine therapy. Transplantation 46:694-703, 1988 31. Vieira JM, Jr., Noronha IL, Malheiros DM, Burdmann EA: Cyclosporine-induced interstitial fibrosis and arteriolar TGF-beta expression with preserved renal blood flow. Transplantation 68:1746-1753, 1999 32. Bertani T, Ferrazzi P, Schieppati A, Ruggenenti P, Gamba A, Parenzan L, Mecca G, Perico N, Imberti O, Remuzzi A, .: Nature and extent of glomerular injury induced by cyclosporine in heart transplant patients. Kidney Int 40:243-250, 1991 33. Myers BD, Sibley R, Newton L, Tomlanovich SJ, Boshkos C, Stinson E, Luetscher JA, Whitney DJ, Krasny D, Coplon NS, .: The long-term course of cyclosporine-associated chronic nephropathy. Kidney Int 33:590-600, 1988 34. Perico N, Remuzzi A, Imberti O, Cavallotti D, Bertani T, Remuzzi G: Morphometrical analysis of glomerular changes induced by cyclosporine in the rat. Am J Kidney Dis 17:537-543, 1991 35. Mihatsch MJ, Antonovych T, Bohman SO, Habib R, Helmchen U, Noel LH, Olsen S, Sibley RK, Kemeny E, Feutren G: Cyclosporin A nephropathy: standardization of the evaluation of kidney biopsies. Clin Nephrol 41:23-32, 1994 36. Joosten SA, van Dixhoorn MG, Borrias MC, Benediktsson H, van Veelen PA, Van Kooten C, Paul LC: Antibody response against perlecan and collagen types IV and VI in chronic renal allograft rejection in the rat. Am J Pathol 160:1301-1310, 2002 37. Habib R, Zurowska A, Hinglais N, Gubler MC, Antignac C, Niaudet P, Broyer M, Gagnadoux MF: A specific glomerular lesion of the graft: allograft glomerulopathy. Kidney Int Suppl 42:S104-11.:S104-S111, 1993 38. Habib R, Broyer M: Clinical significance of allograft glomerulopathy. Kidney Int Suppl 43:S95-8.:S95-S98, 1993 39. Maryniak RK, First MR, Weiss MA: Transplant glomerulopathy: evolution of morphologically distinct changes. Kidney Int 27:799-806, 1985 40. Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Yamaguchi Y, .: The Banff 97 working classification of renal allograft pathology. Kidney Int 55:713-723, 1999 41. Suri DL, Tomlanovich SJ, Olson JL, Meyer TW: Transplant glomerulopathy as a cause of late graft loss. Am J Kidney Dis 35:674-680, 2000 42. Kasiske BL, Kalil RS, Lee HS, Rao KV: Histopathologic findings associated with a chronic, progressive decline in renal allograft function. Kidney Int 40:514-524, 1991 43. Sijpkens YW, Doxiadis II, van Kemenade FJ, Zwinderman AH, de Fijter JW, Claas FH, Bruijn JA, Paul LC: Chronic rejection with or without transplant vasculopathy. Clin Transplant 17:163-170, 2003 44. Dell'Antonio G, Randhawa PS: "Striped" pattern of medullary ray fibrosis in allograft biopsies from kidney transplant recipients maintained on tacrolimus. Transplantation 67:484-486, 1999 45. Bakker RC, Koop K, Sijpkens YW, Eikmans M, Bajema IM, de Heer E, Bruijn JA, Paul LC: Early interstitial accumulation of collagen type I discriminates chronic rejection from chronic cyclosporine nephrotoxicity. J Am Soc Nephrol 14:2142-2149, 2003 |