
|
Paneles de Discussión
Paneais de Discussio |
Schistosomiasis and the Kidney Rashad S. Barsoum MD, FRCP, FRCPE
Professor of Medicine, Cairo University. Cairo. Egypt Schistosomiasis is a parasitic infection that affects 200 million people mainly in Africa, China and South America. It is directly responsible for an annual death of 20,000 in endemic areas. As a cause of chronic renal disease, schistosomiasis is responsible for 20-30 % of patients on regular dialysis in Egypt. Although this proportion is progressively decreasing as a result of successful control programs in that country, schistosomiasis remains as an increasing health hazard in other parts of the world. Of 7 human-pathogenic species, three are responsible for most of morbidity in man: S. hematobium in Africa, S. mansoni in Africa and South America and S. Japonicum in the Far East (figure 1). 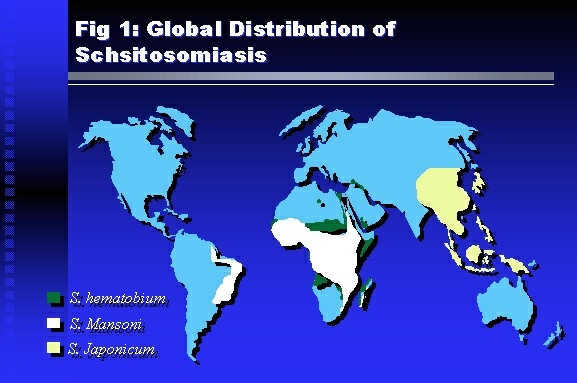 Infection is acquired through contact with infected fresh water of slow-flowing rivers and canals. The infective stage, a bifid-tailed cercaria, penetrates through the skin, matures in the blood stream into schistosomulae, which become bisexual adult worms (Figure 2) in the venules of the liver in S. mansoni and S. japonicum, or the perivesical plexus in S. hematobium. Female worms lay eggs, which penetrate the colonic, rectal of vesical mucosa to reach the exterior along with human excreta. They hatch, releasing very actively moving ciliated miracidia, which find their way to the intermediate host. This is a snail that differs according to species. Through an asexual cycle in the snail, the meracidium develops into a cercaria thereby completing the life cycle. 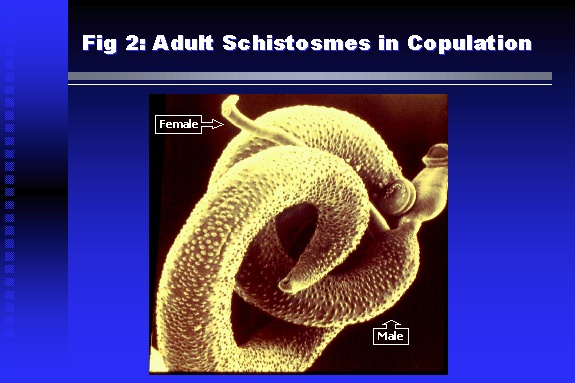 Schistosomiasis is associated with two distinct types of pathology: granulomatous cell-mediated and immune-complex, largely humorally mediated lesions (Figure 3). Both types may be responsible for renal disease in man. 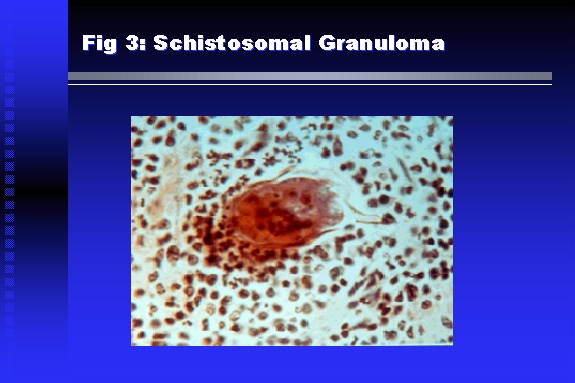 The most common renal lesions are interstitial infection and fibrosis attributed to downstream obstruction, reflux and stone formation. These are the sequelae of fibrosis and calcification of granulomata entrapping S. hematobium ova in the lower urinary tract (Figure 4). The bladder lesions are associated with a high risk of malignancy, usually being of the squamous cell type (Figure 5). 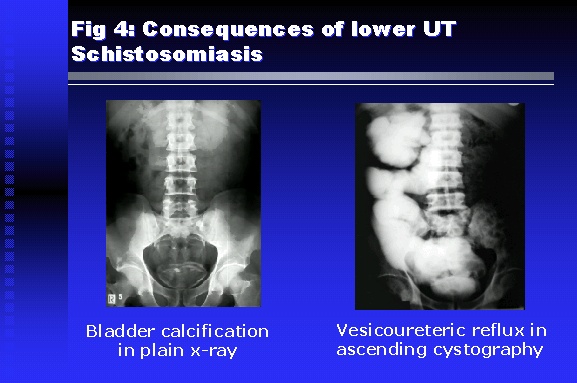 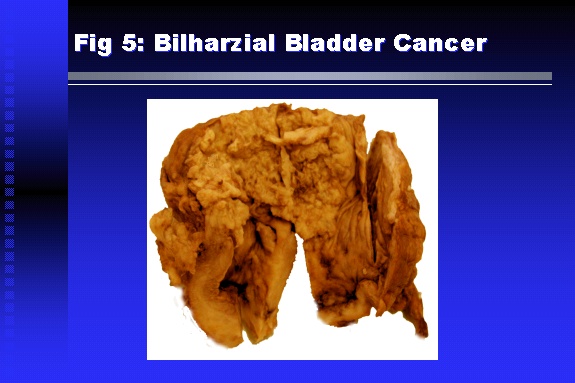 Less frequently, immune complexes containing S. hematobium or S. mansoni worm antigens may deposit in the glomeruli leading to 5 classes of glomerulonephritis (GN): Class I lesions are mesangioproliferative (Figure 6). These are the "pure" schistosomal lesions where parasitic gut antigens are deposited along with IgM antibodies and complement, leading to a mesangial response similar to many other infection-associated glomerulopathies. The clinical profile is dominated by variable degrees of proteinuria, which is rarely of the nephrotic range. Renal function is usually preserved. Class I lesions have been reported to respond to anti-schistosomal chemotherapy. 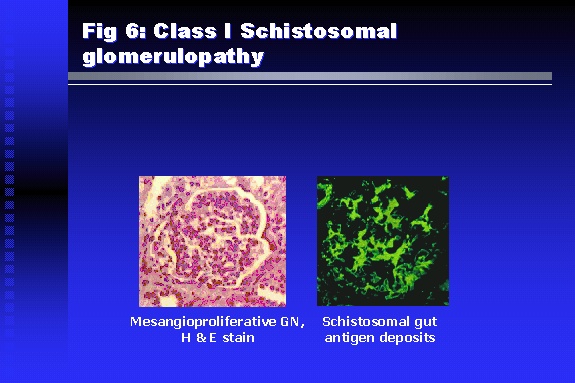 Class II lesions are exudative, with plenty of peripheral blood monocytes and neutrophils infiltrating the glomeruli (Figure 7). This lesion is mostly reported in Africa, being uncommon in South America and unknown in Asia. It is typical of concomitant schistosomal and salmonella infection, a common association in endemic areas that is attributed to the shelter provided to the bacteria by the parasite’s cuticle. Exudative GN usually presents with the nephrotic syndrome, associated with fever, anemia, skin vasculitis and typical hair changes. Response to combined antibacterial and antiparasitic chemotherapy is excellent. 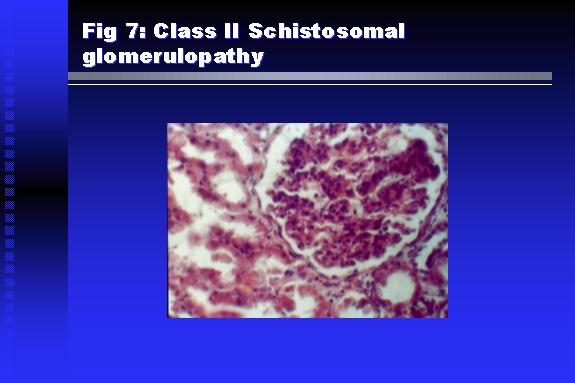 Class III is mesangiocapillary (membranoproliferative) GN (Figure 8). This is the most common lesion associated with symptomatic schistosomal glomerulopathy. It is encountered in about 15 % of patients with advanced S. mansoni-induced hepatic fibrosis with significant portocaval shunts. Proteinuria, often of the nephrotic range, is the usual presentation. Hypertension occurs in 50 % of patients. The lesion is progressive and does not respond to antischistosomal treatment, corticosteroids or immunosuppression. 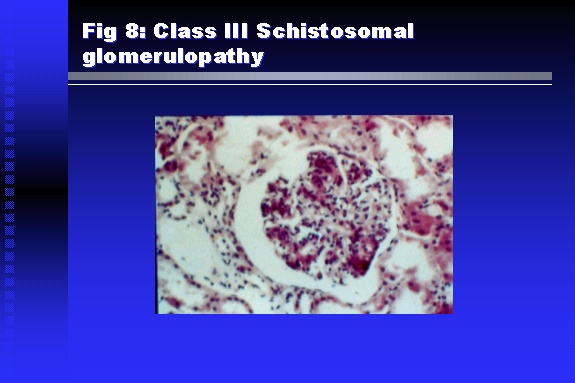 Class III lesions may also occur in concomitant infection with schistosomiasis and hepatitis C, a very common association attributed to epidemiological factors. HCV positive cases are associated with a typical vasculitic rash on the lower limbs, very low serum complement C4 levels, and the presence of hyaline thrombi of cryoglobulins in the glomerular capillaries (Figure 9) 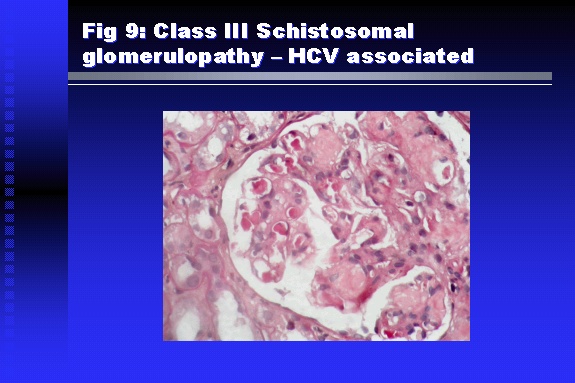 Focal Segmental Sclerosis (Figure 10) is the lesion characterizing Class IV. Like with Class III, it occurs in patients with advanced hepatic fibrosis. It is more frequently encountered in the black populations in Africa and Brazil. The clinical presentation is indistinguishable from that seen with Class III. Likewise, it does not respond to any form of therapy. 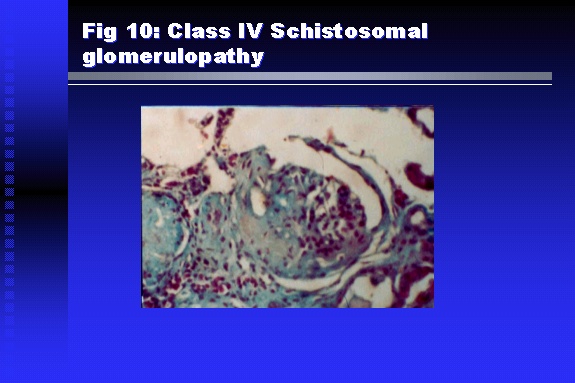 Both Classes III and IV are characterized by IgA glomerular deposits in m ore than half of cases. Serum total IgA levels may be increased, but more consistently so are antigliadin IgA antiobodies (Figure 11). There is evidence that mucosal IgA production is increased due to the local colonic granulomata, while its clearance is impaired due to encroachment on hepatic macrophage activity by the fibrotic process. More recent evidence suggests that B-lymphocytes may be switched to produce more IgA in response to schistosomal or other associated infections, under the influence of the Th2-cytokines particularly IL-10, supervening in late schistosomal lesions. An autoimmune process has also been suggested to propagate schistosomal lesions, both in IgA positive and IgA negative cases (Figure 12). 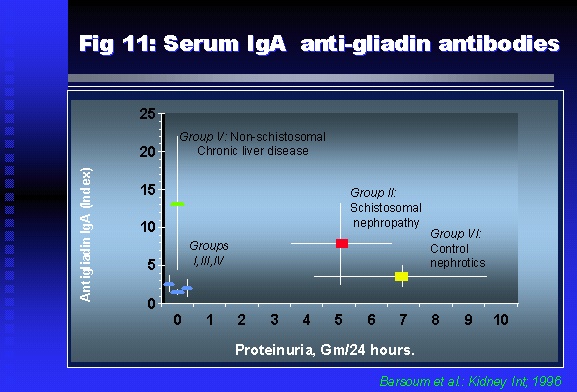 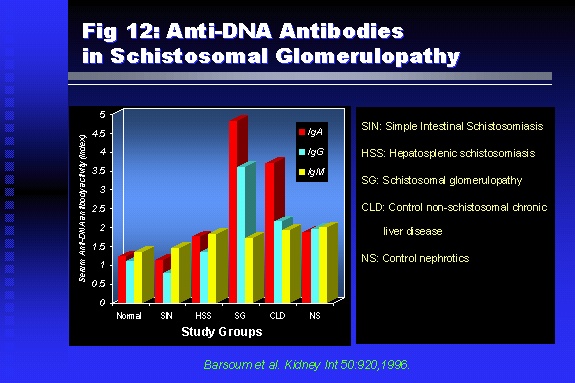 Renal amyloidosis constitutes Class IV schistosomal glomerulopathy (Figure 13). It is encountered with almost equal frequency in S. hematobium and S. mansoni infections. Amyloid deposits are of the AA type, and may be also found in the liver. When searched by special stains, Amyloid deposits may be detected in 15 % of all biopsies obtained from patients with schistosomal nephropathy. The clinical presentation of such cases is indistinguishable from earlier classes. Schistosomal Amyloid lesions do not respond to any form of treatment. 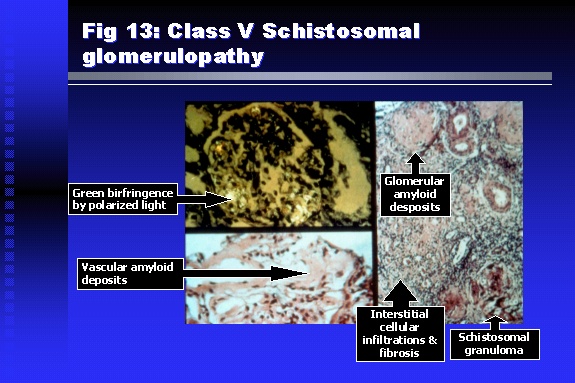 In addition to its important epidemiological and clinical impact, Schistosomal kidney disease has provided an excellent experimental and clinical model of glomerular injury, which continues to enrich our information on the pathogenesis of many forms immune mediated glomerulonephritis. Further reading: Barsoum RS (1993). Schistosomal glomerulopathies. Kidney Int 44(1): 1-12 Barsoum RS, Nabil M, Saady G, Et Al (1996): Immunoglobulin A and the pathogenesis of schistosomal glomerulopathy. Kidney Int 50(3): 920-928 Barsoum R. (1998). Schistosomiasis. In: Oxford Textbook of Clinical Nephrology. Ed. A.M. Davison, J.S. Cameron, J.P. Grunfeld, D.N.S. Kerr, E. Ritz, C.G. Winearls. Oxford University Press, Oxford, New York, Toronto. Ed. II, p1287-1302. Barsoum, R. (2000). The Kidney in Schistosomal infection. In: Textbook of Nephrology. Ed. SG Massry & RJ Glassock. Lippincott Williams & Wilkins, Philadelphia, Baltmore, New York, London, Buenos Aires, Hong Kong, Sydney, Tokyo. Ed. IV. pp. 680-4 Barsoum, R. (2000). Urinary Schistosomiasis. In: Comprehensive Clinical Nephrology. Ed. R.J. Johnson, J. Feehally. Mosby London, Edinburgh, New York, Philadelphia, St. Louis, Sydney, Toronto. pp. 10.59.1-6 Seminars Barsoum R (2003). Schistosomiasis and the kidney. Semin Nephrol. 23(1):34-41. |