
|
Paneles de Discussión
Paneais de Discussio |
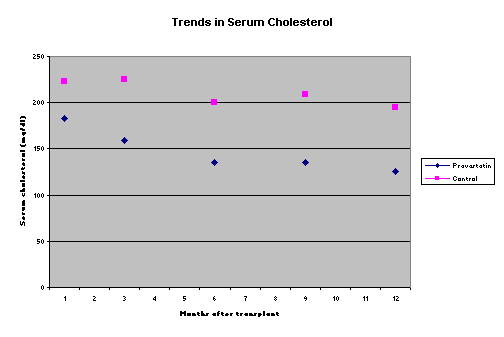
Role of pravastatin in pediatric renal transplantationLavjay Butani, M.D.Section of Pediatric Nephrology. University of California Davis Medical Center Sacramento, California. USAlavjay.butani@ucdmc.ucdavis.eduIntroduction Hyperlipidemia is a common occurrence both in adult and pediatric renal transplant recipients with a reported prevalence of 30%-75%, even on long-term follow-up1,2. In fact, with the growing popularity of Sirolimus in transplant recipients, hyperlipidemia may become an even bigger problem. Persistent dyslipidemia can eventually lead to accelerated atherosclerosis and ischemic heart disease3, which is already more common to begin with in the chronic renal failure cohort. In actual fact, cardiovascular diseases leading to death are the commonest cause of allograft loss in the transplant population. There is also growing concern in the transplant community that a high-risk lipid profile could promote allograft injury, contributing to the development and progression of chronic allograft nephropathy4. As a consequence, interest in monitoring and attempting to prevent and to treat the hyperlipidemia in the post-transplant period has increased dramatically. While dietary modification, to reduce the intake of saturated fats, consistent aerobic exercise, and weight loss (for the overweight patient) should certainly be strongly recommended to all patients after renal transplantation, the practical benefit of these life-style changes is quite limited. Delucchi et al offered the Step II American Heart Association diet (containing a low fat and low saturated cholesterol content) to 22 children with hyperlipidemia after renal transplantation; only about half of the eligible children agreed to participate in the study. Moreover, no patient demonstrated 100% compliance with the diet. Even in the setting of such a controlled study, the benefit of the diet in ameliorating the dyslipidemia was quite modest. No patient lost weight, nor was the mean body mass index affected, and while the total cholesterol and LDL-cholesterol did decrease, the magnitude of the decline was small (11% and 14% respectively at 12 weeks)5. While other maneuvers such as corticosteroid minimization and corticosteroid avoidance or withdrawal may be of benefit in reducing lipid levels, such interventions are not without risk, especially of acute rejection. A safer alternative available to transplant nephrologists is the use of lipid lowering agents such as the HMGCoA-reductase inhibitors. This could be done either pre-emptively from the immediate post-transplant period in all patients, or alternatively only in the post-transplant patients who develop persistent hyperlipidemia. While several adult studies have been published describing the benefits of either approach, little data are available in the pediatric transplant population. Brief overview of the HMGCoA reductase inhibitors and their use in adult transplant recipients The HMGCoA reductase inhibitors, commonly referred to as the ‘statins’, are a group of structurally different compounds, some natural and some purely synthetic, that inhibit the conversion of HMGCoA to mevalonic acid. Since cholesterol biosynthesis accounts for most of the circulating cholesterol in humans, inhibition of this rate-limiting step leads to lower serum total cholesterol. This reduction in total cholesterol results in an increase in the expression of surface LDL receptors, thereby causing a decrease in serum LDL cholesterol levels. The statins also reduce VLDL synthesis and promote its catabolism, thereby reducing serum VLDL cholesterol too. Beneficial effects of the statins on lipid profile have been demonstrated in adult renal transplant recipients in several studies; the mean reduction in total cholesterol and LDL cholesterol has been shown to be in the range of 20%-30% and 35-40% respectively6,7. A few studies have also demonstrated a reduction in the total triglyceride level with higher doses of the statins7,8; no significant change in the HDL cholesterol has been seen with these agents6, 7, 9. In fact, a recently published randomized double blind study of fluvastatin versus placebo in hypercholesterolemic renal transplant recipients also demonstrated a benefit of fluvastatin in reducing cardiac deaths or non-fatal myocardial infarctions after a mean follow-up of 5 years10. A burgeoning area of interest as well as ongoing research in the transplant literature relates to the immunomodulatory and anti-inflammatory effects of the statins. This has recently been reviewed by Palinski et al11. To summarize the data in this regard, the statins may modulate the immune system by a) inhibiting the expression of the class-II major histocompatibility antigens on non-professional antigen presenting cells, thereby reducing T cell proliferation, b) blocking the leukocyte functioning antigen-1, which is a co-stimulator of T cells and is also involved in T-cell adhesion, c) increasing nitric oxide generation, and d) inhibiting the post-translational prenylation of a variety of proteins, including leukocyte adhesion molecules. Other effects of the statins that may be of value in the transplant population include an increase in fibrinolysis and inhibition of smooth muscle proliferation. While the in-vitro data clearly support such an immunomodulatory effect, clinical trials with the statins have not shown a consistent beneficial effect. The use of pravastatin has been associated with an improved allograft survival and a lower incidence of severe cardiac rejection in a single study of cardiac transplant recipients12. Similarly, a pilot study in renal transplant recipients showed that the use of pravastatin lowered the incidence of biopsy-proven acute rejection13. However, at this time, other investigators have not been able to confirm these results8, 14. Pediatric studies on the efficacy of the ‘statins’ in transplant recipients Statins have been used safely and successfully in improving the lipid profile in children and adolescents with familial hypercholesterolemia15, but data on their use in pediatric transplant recipients are limited. Penson et al were the first to demonstrate the efficacy of pravastatin in ameliorating hypercholesterolemia in 21 pediatric and adolescent heart transplant recipients with no adverse consequences16. In the past 2 years, three groups of investigators, including our own, have published data on the use of the statins in children with renal transplants. Krmar et al used low-dose atorvastatin in 8 children and young adults who were renal transplant recipients and who had inadequately controlled hypercholesterolemia; at the end of the study, the total serum cholesterol was lowered by 32% and the LDL-cholesterol by about 40%17. These investigators also noted a decrease in the daytime and nighttime systolic and diastolic blood pressure values (determined during ambulatory monitoring) in a subset of the treated patients. Subsequently Argent et al, in a prospective study, showed that atorvastatin safely reduced total cholesterol, LDL cholesterol and serum triglycerides by approximately 40%, 60% and 45% respectively, in 9 children with renal transplants who had persistent hyperlipidemia18. Our own study was quite different from the previously published pediatric data in that, based on our center’s favorable experience using pravastatin pre-emptively in adult renal transplant patients13 we routinely started using pravastatin pre-emptively in all pediatric renal transplant patients from June 1999 onwards19. Seven children who had received pravastatin from the immediate post-transplant period were compared to the 9 previous consecutively transplanted pediatric patients who had not received pravastatin (the control group). All patients received our same standard dietary instructions, with recommendations to reduce the intake of salt, concentrated refined sugars and saturated fats. All patients received triple immunosuppression with tapering doses of corticosteroids, azathioprine or mycophenolate mofetil, and either cyclosporine or tacrolimus. A standard fixed dose of pravastatin was used; children <10 yr of age received 10 mg once daily while those >10 yr were treated with 20 mg once daily. Pravastatin was initiated within 24–48 h of transplantation. Neither the baseline demographic characteristics nor the mean pre-transplant serum cholesterol were significantly different between the control and treatment groups. As can be seen in Figure 1, the serum cholesterol in the pravastatin group was significantly lower at all time points except at 1 month post-transplant. The repeated measures ANOVA test showed that in the pravastatin group there was a significant decline in the serum cholesterol at 1, 3, 6, 9 and 12 months after transplantation compared to the baseline value (p < 0.05 for each comparison); no such difference was seen in the serum cholesterol in the control patients. Although there were some differences between the 2 groups with respect to the cumulative corticosteroid exposure, such a difference was seen only at 3 and 6 months. Our study has now been expanded to include data collection on a complete lipid profile (total, LDL-, VLDL- and HDL- cholesterol, and triglycerides) in a prospective manner in all children transplanted at our center. Recruitment is still ongoing. No pediatric study has adequately evaluated the effect of statins on acute rejection and long-term graft survival. Figure 1
 Risks of statins The main concern with the use of the statins, especially in the transplant population, relates to the potential for rhabdomyolysis20. The mechanism underlying this myopathy is related to the inhibition of the mevalonic pathway by the statins in skeletal muscle cells. The lipophilic statins, such as lovastatin, by virtue of their ability to cross the cell membrane and gain access to the skeletal muscle cells in larger concentrations, are associated with the highest risk of rhabdomyolysis. This risk is compounded even further when other drugs that share the metabolic pathway of the statin in question are co-administered, resulting in very high plasma levels of the statin. As an example, administration of the calcineurin inhibitors (cyclopsorine and tacrolimus), along with lovastatin, both of which are metabolized by the cytochrome P450 3A (CYP 3A) system, greatly increases the risk of myopathy. On the other hand statins such as pravastatin or fluvastatin, which are not metabolized by the CYP 3A are not associated with an increased risk when administered to transplant recipients who are taking calcineurin inhibitors. Other adverse effects of the statins that physicians and patients need to be aware of are:
None of the pediatric patients in the aforementioned studies developed any adverse events to the statins. There were no elevations in the creatine kinase level, nor did any patients complain of myalgias or have muscle tenderness on examination. Conclusions In conclusion:
References
|