
|
Paneles de Discussión
Paneais de Discussio |
IL-2 ANTIBODY INDUCTION ON PEDIATRIC RENAL TRASPLANTSClotilde Druck Garcia, Viviane Rocha de Barros, Valter Duro GarciaPediatric Nephrology Unit- Hospital da Criança Santo Antônio
|
|
Author |
Induction |
Immunosuppressor |
N |
Incidence of Acute rejection (%) 6 mo |
|
Garcia (10) |
Basiliximab Daclizumab |
Cyclosporin Tacrolimus MMF or Aza |
174 |
17,8 |
|
Garcia(11) |
Basiliximab Daclizumab |
Cyclosporin Tacrolimus MMF or Aza |
34 |
17,6 |
|
Montini(12) |
Basiliximab |
Tacrolimus |
42 |
12 |
|
Ferraris(13) |
Basiliximab |
Cyclosporin |
18 (LRD) |
0 |
|
Machado(14) |
Basiliximab |
Cyclosporin Tacrolimus |
22 |
23 |
|
Pape(15) |
Basiliximab |
Cyclosporin |
48 |
14 |
|
Swiatecka-Urban (16) |
Basiliximab |
Tacrolimus |
24 |
26 |
|
Offner (17) |
Basiliximab |
Cyclosporin |
41 |
15 |
|
Vester(18) |
Cyclosporin |
38 |
21 |
Dose:
The dose of basiliximab in children difers from the used in adults. In the beginning, two intravenous (IV) bolus injections of basiliximab (12 mg/m2) were administered (before and 4 days postsurgery). Later, two injections in patients less than 40 kg, 10 mg and in patients over 40 kg, 20 mg are administered at the same time points. (17, 19)
In phase III trials daclizumab was used in a five-dose regimen of 1 mg/kg at 2-weekly intervals, resulting in saturation of IL-2Ralpha on circulating lymphocytes for up to 120 days after renal transplantation. One study with the purpose to evaluate daclizumab blood concentrations and the saturation of the IL-2Ralpha on the circulating lymphocytes with a limited dosing regimen of daclizumab. Twelve patients undergoing primary cadaver or living donor transplantation were randomized to either receive one dose (2 mg/kg) or two doses (2nd dose, 1 mg/kg) of daclizumab in addition to maintenance immunosuppression therapy. Pharmacokinetic and pharmacodynamic studies were performed up to 20 weeks after the transplantation. In patients treated with a single dose of daclizumab, the blood concentrations of daclizumab declined to 1  g/mL at 43±
7 days after the transplantation. In patients treated with two doses of daclizumab, the blood concentrations of daclizumab declined to 1 micro g/mL at 45 ±
13 days after the second dose for a total of 59 ± 13 days after the transplantation. Daclizumab levels of 1
g/mL at 43±
7 days after the transplantation. In patients treated with two doses of daclizumab, the blood concentrations of daclizumab declined to 1 micro g/mL at 45 ±
13 days after the second dose for a total of 59 ± 13 days after the transplantation. Daclizumab levels of 1 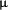 g/mL or greater were associated with saturation of the IL-2Ralpha on the circulating lymphocytes. In the new era of effective maintenance immunosuppression, a limited dosing regimen of daclizumab may be desired, practical and economical (20).
g/mL or greater were associated with saturation of the IL-2Ralpha on the circulating lymphocytes. In the new era of effective maintenance immunosuppression, a limited dosing regimen of daclizumab may be desired, practical and economical (20).
Adverse effects:
Basiliximab and daclizumab are potent and relatively safe immunosuppressive induction agents used in transplantation. But there are some data showing cases with adverse effects. Leonard et al. report (21) a 42-year-old woman who received a dose of basiliximab without adverse reaction before a previous renal transplant that was canceled. Two weeks later, she received a second dose of basiliximab. Within 10 min of receiving the second dose, she developed chest tightness, shortness of breath, tongue swelling, diffuse pruritic rash, and skin flushing. The authors hypothesized that her anaphylaxis was mediated by IgE antibodies to basiliximab. Consistent with this hypothesis, intradermal administration of a 1:100 dilution of basiliximab induced a 10 x 10-mm flare. She mounted neither a prick nor an intradermal response to daclizumab. The patient was administered daclizumab without any adverse effects. The negative skin test and safe administration of daclizumab is surprising because the similarity of these hybrid antibodies would have predicted similar IgE responsiveness and clinical outcome. The authors propose that patients who develop anaphylaxis to basiliximab or other chimeric antibodies may be candidates for treatment with a humanized antibody preparation such as daclizumab in the presence of a negative skin test to the humanized agent. There are other reports of anaphylatic shock, including pediatric patients (22, 23, 24).
Costs
Costs in renal transplant recipients treated with basiliximab or placebo plus triple immunosuppressive therapy are similar. Considering the sum of costs regarding number of days spent in the hospital, steroid pulses, OKT3 treatment, graft biopsies, dialysis sessions in patients with acute rejection compared with a significant reduction in rejection episodes and a shorter length of stay in hospital achieved with basiliximab or daclizumab there was no statistically significant economical difference(25, 26).
Conclusion
Monoclonal antibody therapy (daclizumab and basiliximab) is an exciting new development, whereby T cell proliferation is inhibited by selective blockade of interleukin (IL)-2 receptors. Further clinical trials need to be carried out to establish efficacy, tolerability and pharmacokinetics in pediatric transplant recipients. Preliminary results, when used in combination with a standard immunosuppressive regimen, are good with respect to incidence of acute graft rejection, host immune response and adverse effects.
References
1. Shapiro R. Pediatric renal transplantation: review of recent literature. Curr Opin Organ Transplant 2000, 5: 324-329.
2. Tejani A, Sullivan EK. The impact of acute rejection on chronic rejection. A report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Transplant 2000, 4: 107-111.
3. Morgan D, Ruscettis F, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrow. Science 1976, 193: 1007-1008.
4. Robb R, Greene W, Rusk C. Low and high affinity cellular receptors for interleukin 2: implications for the level of Tac antigen. J Exp Med 1984, 162: 358-362.
5. Kirkman RL, Shapiro ME, Carpenter CB, McKay DB, Milford EL, Ramos EL, Tilney NL, Waldmann TA, Zimmerman CE, Strom TB. A randomised trial of anti-Tac monoclonal antibody in human renal transplantation. Transplantation 1991, 51: 107-113
6. Van Gelder T, Zietse R, Mulder AH, Yzermans JN, Hesse CJ, Vaessen LM, Weimar W. A double blind, placebo-controlled study of monoclonal anti-interleukin-2 receptor antibody (BT563) administration to prevent acute rejection after kidney transplantation. Transplantation 1995, 60: 248-252.
7. Olyaei AJ, Thi K, deMattos AM, Bennett WM. The use of basiliximab and daclizumab in kidney transplantation. Prog Transplant 2001, 11:33-7
8. Matas A, Gillingham K, Payne W, Najarian J. The impact of an acute rejection episode on long-term renal allograft survival. Transplantation 1994, 57: 857-859.
9. Adu D, Cockwell P, Ives NJ, Shaw J, Wheatley K. Interleukin-2 receptor monoclonal antibodies in renal transplantation: meta-analysis of randomised trials. BMJ 2003, 326:789
10. Garcia CD, Koch-Nogueira PC, Machado PP, Meneses RP, Lima EM, Penido JM, Barros VR, Araújo LMP, David-Neto E. IL-2 receptor antibody (IL2RAb) induction and acute rejection in pediatric renal transplant- data from the Brazilian Pediatric Renal transplant Registry. Pediatr Transplant 2003, Suppl 4: S58
11. Garcia CD, Schneider LH, Barros VR, Guimarães PC, Garcia VD. Pediatric renal transplantation under tacrolimus or cyclosporine immunosuppression and basiliximab induction. Transplant Proc 2002, 34:2533-4.
12. Montini G, Murer L, Pietrobon B , Ghio L, Ginevri F, Cardillo M, Scalamogna M, Edefonti A, Perfumo F, Zacchello G. Trial on the use of tacrolimus and basiliximab in kidney transplantation: preliminary data. Pediatr Nephrol 2002; 17:C57
13. Ferraris J, Tambutti M, Redal M et al. Daclizumab as induction therapy in pediatric renal transplantation: results after six months of treatment. Pediatr Nephrol 2002; 16:C176
14. Machado PGP, Takabatake E, Nogueira PCK, Feltran LS, Carvalhaes JT, Silva Jr HT, Medina JOPl. Basiliximab produces better graft function after kidney transplantation in children. Pediatr Nephrol 2001; 16:C175
15. Pape L, Strehlau J, Henne T, Latta K, Nashan B, Ehrich JH, Klempnauer J, Offner G. Single centre experience with basiliximab in paediatric renal transplantation. Nephrol Dial Transplant. 2002, 17:276-80
16. Swiatecka-Urban A, Garcia C, Feuerstein D, Suzuki S, Devarajan P, Schechner R, Greenstein S, Tellis V, Kaskel F. Basiliximab induction improves the outcome of renal transplants in children ans adolescents. Pediatr Nephrol 2001, 16:693-696.
17. Offner G, Broyer M, Niaudet P, Loirat C, Mentser M, Lemire J, Crocker JF, Cochat P, Clark G, Chodoff L, Korn A, Hall M. A multicenter, open-label, pharmacokinetic/pharmacodynamic safety, and tolerability study of basiliximab (Simulect) in pediatric de novo renal transplant recipients. Transplantation. 2002, 74:961-6.
18. Vester U, Kranz B, Testa G, Malago M, Beelen D, Broelsch CE, Hoyer PF. Efficacy and tolerability of interleukin-2 receptor blockade with basiliximab in pediatric renal transplant recipients Pediatr Transplant 2001, 5:297-301.
19. Kovarik JM, Offner G, Broyer M, Niaudet P, Loirat C, Mentser M, Lemire J, Crocker JF, Cochat P, Clark G, Gerbeau C, Chodoff L, Korn A, Hall M. A rational dosing algorithm for basiliximab (Simulect) in pediatric renal transplantation based on pharmacokinetic-dynamic evaluations. Transplantation. 2002, 74:966-71.
20. Vincenti F, Pace D, Birnbaum J, Lantz M. Pharmacokinetic and pharmacodynamic studies of one or two doses of daclizumab in renal transplantation. Am J Transplant 2003, 3:50-2
21. Leonard PA, Woodside KJ, Gugliuzza KK, Sur S, Daller JA. Safe administration of a humanized murine antibody after anaphylaxis to a chimeric murine antibody. Transplantation 2002, 74:1697-700.
22. Baudouin V, Crusiaux A, Haddad E et al. Anaphylactic shock caused by immunoglobulin E sensitization after retreatment with the chimeric anti-interleukin-2 receptor monoclonal antibody basiliximab. Transplantation. 2003;76:459-63.
23. Bamgbola FO, Del Rio M, Kaskel FJ, Flynn JT. Non-cardiogenic pulmonary edema during basiliximab induction in three adolescent renal transplant patients. Pediatr Transplant 2003, 7:315-20.
24. Barros VR, Rocha V, Garcia VD, Garcia CD. Anaphylactic shock after retreatment with basiliximab(*). Transplant Proc. 2003, 35:579.
25. Walters SJ, Whitfield M, Akehurst RL, Chilcott JB. Economic implications of the use of basiliximab in addition to triple immunosuppressive therapy in renal allograft recipients: a UK perspective. Pharmacoeconomics 2003, 21:129-38.
26. Nogueira PK, Feltran LS, Tabatabake E, Machado PP, Carvalhaes JTA, Pestana JOM. Basiliximab does not increase costs of kidney transplantation in children. Pediatr Nephrol 2001; 16:C176