
|
Paneles de Discussión
Paneais de Discussio |
Chronic viral hepatitis infection in patients on renal replacement therapyThomas Fehr and Patrice M. AmbühlDivision of Nephrology, Department of Internal Medicine,
thomas.fehr@tbrc.mgh.harvard.edu |
|
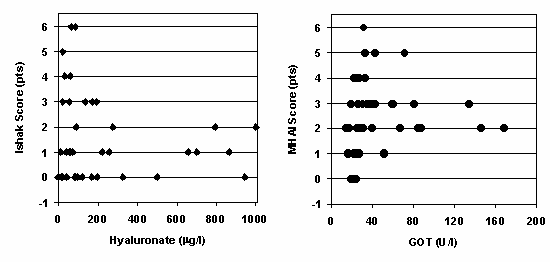
Figure 1. Correlation of histological and biochemical parameters in 41 renal allograft recipients with chronic HBV or HCV infection [11]. |
 |
|
Left panel:Correlation of the fibrosis marker hyaluronate with the histological score of liver fibrosis according to Ishak (scores of 5 and 6 reflect focal and diffuse cirrhosis, respectively; 6 is the maximum score).
Right panel: Correlation of serum glutamate-oxalacetate transaminase (GOT) concentration at the time of biopsy with the histological score of necroinflammatory liver lesions (MHAI; maximum score is 18). |
HBV and HCV infection harbour the risk of developing hepatocellular carcinoma. Therefore, and irrespective of therapeutic decisions, these patients should be followed by yearly sonogaphic evaluation of the liver and determination of alpha-fetoprotein every 6 months for early diagnosis of this otherwise inevitably fatal malignancy.
Therapeutic approach
General remarks: role and risk of interferon therapy
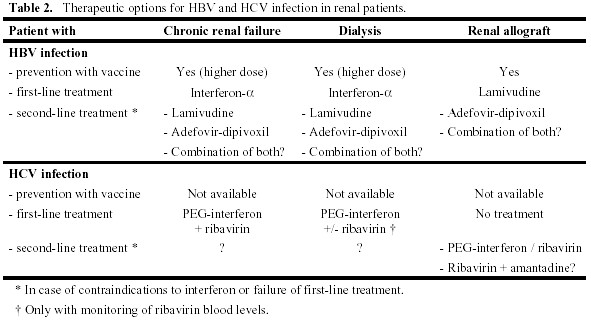
An overview of therapeutic options for hepatitis virus infections in renal patients is given in Table 2. The standard therapies for HBV and HCV infection involve application of interferon-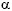 . The main side effects of this treatment are a flu-like syndrome, myelotoxicity with development of single or multiple lineage cytopenias and neurologic/psychiatric symptoms. Therefore, patients with a neurologic and/or psychiatric disorder are usually excluded from therapy as are patients with anaemia, leukopenia and thrombocytopenia. All patients with advanced renal failure suffer from hyporegenerative anaemia, and some may have thrombocytopenia in the context of the primary disease and its treatment (e.g. systemic lupus erythematosus). Therefore, aggressive treatment of anaemia with erythropoietin, iron and vitamins should be performed in renal patients before beginning a therapy with interferon. When patients with autoimmune disorders are treated with interferon, they should be meticulously followed for symptoms and signs of their primary disease, since interferon may cause a flare-up. However, for virus-induced autoimmunity (such as HCV-mediated cryoglobulinaemia) antivirals are the mainstay of specific treatment.
. The main side effects of this treatment are a flu-like syndrome, myelotoxicity with development of single or multiple lineage cytopenias and neurologic/psychiatric symptoms. Therefore, patients with a neurologic and/or psychiatric disorder are usually excluded from therapy as are patients with anaemia, leukopenia and thrombocytopenia. All patients with advanced renal failure suffer from hyporegenerative anaemia, and some may have thrombocytopenia in the context of the primary disease and its treatment (e.g. systemic lupus erythematosus). Therefore, aggressive treatment of anaemia with erythropoietin, iron and vitamins should be performed in renal patients before beginning a therapy with interferon. When patients with autoimmune disorders are treated with interferon, they should be meticulously followed for symptoms and signs of their primary disease, since interferon may cause a flare-up. However, for virus-induced autoimmunity (such as HCV-mediated cryoglobulinaemia) antivirals are the mainstay of specific treatment.
Special emphasis should be given to renal allograft recipients with replicating HBV and HCV infection. Several reports of small case series indicate that interferon as non-specific immunostimulatory agent causes acute often humoral allograft rejection in about 20-30% of patients [14-16]. Therefore, interferon should be avoided in this patient population. However, a closer look reveals that rejection could be successfully treated in some cases by immediate stop of interferon and standard rejection treatment including steroids and anti-T cell antibodies [17]. Based on these findings we suggest to chose primarily a therapeutic strategy without interferon in renal transplant patients. In particularly desperate cases, interferon could be offered to a patient after open discussion on the risks of allograft rejection with subsequent intensified immunosuppressive treatment and potential graft loss [18]. These patients should be very closely monitored for acute allograft rejection during this treatment.
Specific therapeutic options in different patient groups
HBV infection. Mainstay of treatment of this disease is prevention! A highly efficient recombinant vaccine for HBV is available and has been effective also in renal patients. However, the response rate in patients with advanced renal failure and patients on dialysis is clearly lower [19]. Therefore, vaccination should be performed at an early stage of moderate renal failure (creatinine clearance > 40 ml/min). For patients with advanced renal failure and haemodialysis patients, a special vaccine with a four-fold higher dose can be used to obtain a better response rate.
In case of established replicating HBV infection with histological alterations antiviral therapy should be considered. In patients with advanced renal failure and in dialysis patients – especially transplant candidates – a therapeutic trial with interferon-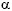 should be performed, since it offers the highest efficacy. In case of treatment failure or serious contraindications, the antiviral drugs lamivudine and adefovir-dipivoxil can be used [20]. Whereas lamivudine has been a standard treatment for years, adefovir-dipivoxil has recently proven to be effective against lamivudine-resistant HBV. Combination treatment has equal primary response rates, but a lower rate of therapy failure due to lamivudine resistance than monotherapy with lamivudine. However, no controlled trials have been performed with adefovir-dipivoxil in patients with advanced renal failure or renal allograft recipients. HBV pre-core mutants (HBeAg-negative) are especially difficult to treat. Those patients as well as patients scheduled for renal transplantation with contraindications for interferon-
should be performed, since it offers the highest efficacy. In case of treatment failure or serious contraindications, the antiviral drugs lamivudine and adefovir-dipivoxil can be used [20]. Whereas lamivudine has been a standard treatment for years, adefovir-dipivoxil has recently proven to be effective against lamivudine-resistant HBV. Combination treatment has equal primary response rates, but a lower rate of therapy failure due to lamivudine resistance than monotherapy with lamivudine. However, no controlled trials have been performed with adefovir-dipivoxil in patients with advanced renal failure or renal allograft recipients. HBV pre-core mutants (HBeAg-negative) are especially difficult to treat. Those patients as well as patients scheduled for renal transplantation with contraindications for interferon-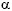 should primarily be evaluated together with an experienced hepatologist. The risk of replicating HBV infection has to be balanced carefully to the risk of developing lamivudine resistance and therefore losing a treatment option for the time after transplantation.
should primarily be evaluated together with an experienced hepatologist. The risk of replicating HBV infection has to be balanced carefully to the risk of developing lamivudine resistance and therefore losing a treatment option for the time after transplantation.
For patients with a renal allograft we support first-line antiviral treatment with lamivudine, therefore avoiding the risk of rejection by interferon. A recent report has shown an excellent response rate (biochemical response 80-100%, virological response 67-100%; [21]). In case of treatment failure or development of lamivudine resistance, a trial with adefovir-dipivoxil or a combination of both antivirals can be performed, and the patient should be followed together with an experienced hepatologist.
HCV infection. Therapy of HCV infection in renal allograft recipients is difficult, since interferon should be avoided and other therapy regimens (mainly ribavirin alone) have failed to induce a sustained virological response or amelioration of liver histology [22]. However, our recent report of liver biopsy results in 23 long-term HCV-infected renal allograft recipients showed that none of them had developed cirrhosis after a mean follow-up of 12 years after renal transplantation [11]. It indicates that part of the liver tissue damage may be induced by immunopathology and not by HCV directly [23], and hence may even improve under immunosuppressive therapy. Therefore, renal allograft recipients with replicating HCV infection should not receive therapy to date except patients included in clinical studies and individual cases with very aggressive disease. These should be followed together with a hepatologist, and interferon may be offered under close monitoring of renal function [18].
Treatment of HCV infection should therefore be attempted in the pre-transplant period. Patients with moderate to severe renal failure can be treated according to the standard treatment protocols, which at the moment consist of PEG-interferon and ribavirin [24]. In dialysis patients ribavirin should only be given, when blood levels can be monitored due to a high risk of haemolytic anaemia if overdosed [25, 26]. When interferon treatment alone is used, the rate of sustained virological response is lower. However, two recent reports showed a response rate of 40-60% in this population. All patients that were subsequently transplanted, remained HCV RNA PCR-negative indicating definitive cure of disease [27], and only one developed recurrent glomerulonephritis [28].
Key messages
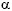 is the first-line treatment in patients with chronic renal failure and patients on dialysis, but should be avoided as a first-line treatment in renal allograft recipients due to a high rate of allograft rejection.
is the first-line treatment in patients with chronic renal failure and patients on dialysis, but should be avoided as a first-line treatment in renal allograft recipients due to a high rate of allograft rejection. 1. Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, Allon M, Bailey J, Delmez JA, Depner TA, Dwyer JT, Levey AS, Levin NW, Milford E, Ornt DB, Rocco MV, Schulman G, Schwab SJ, Teehan BP, Toto R: Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 347:2010-2019, 2002
2. Pascual M, Theruvath T, Kawai T, Tolkoff-Rubin N, Cosimi AB: Strategies to improve long-term outcomes after renal transplantation. N Engl J Med 346:580-590, 2002
3. Denton MD, Singh AK: Recurrent and de novo glomerulonephritis in the renal allograft. Semin Nephrol 20:164-175, 2000
4. Burdick RA, Bragg-Gresham JL, Woods JD, Hedderwick SA, Kurokawa K, Combe C, Saito A, LaBrecque J, Port FK, Young EW: Patterns of hepatitis B prevalence and seroconversion in hemodialysis units from three continents: the DOPPS. Kidney Int 63:2222-2229, 2003
5. Huraib SO: Hepatitis C in dialysis patients. Saudi Med J 24 Suppl 2:S123, 2003
6. Roth D: Hepatitis C virus: the nephrologist's view. Am J Kidney Dis 25:3-16, 1995
7. Hayashi PH, Flynn N, McCurdy SA, Kuramoto IK, Holland PV, Zeldis JB: Prevalence of hepatitis C virus antibodies among patients infected with human immunodeficiency virus. J Med Virol 33:177-180, 1991
8. Kowdley KV, Subler DE, Scheffel J, Moore B, Smith H: Hepatitis C virus antibodies in systemic lupus erythematosus. J Clin Gastroenterol 25:437-439, 1997
9. Abdel-Hamid M, El Daly M, El Kafrawy S, Mikhail N, Strickland GT, Fix AD: Comparison of second and third generation enzyme immunoassays for detecting antibodies to hepatitis C virus. J Clin Microbiol 40:1656-1659, 2002
10. Hanuka N, Sikuler E, Tovbin D, Mostoslavsky M, Hausman M, Orgel M, Yaari A, Shemer-Avni Y: Hepatitis C virus infection in renal failure patients in the absence of anti-hepatitis C virus antibodies. J Viral Hepat 9:141-145, 2002
11. Fehr T, Riehle HM, Nigg L, Gruter E, Ammann P, Renner EL, Ambuhl PM: Evaluation of hepatitis B and hepatitis C virus-infected renal allograft recipients with liver biopsy and noninvasive parameters. Am J Kidney Dis 42:193-201, 2003
12. Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T: Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet 357:1069-1075, 2001
13. Mannucci PM, Remuzzi G, Pusineri F, Lombardi R, Valsecchi C, Mecca G, Zimmerman TS: Deamino-8-D-arginine vasopressin shortens the bleeding time in uremia. N Engl J Med 308:8-12, 1983
14. Rostaing L, Izopet J, Baron E, Duffaut M, Puel J, Durand D: Treatment of chronic hepatitis C with recombinant interferon alpha in kidney transplant recipients. Transplantation 59:1426-1431, 1995
15. Durlik M, Gaciong Z, Rowinska D, Rancewicz Z, Lewandowska D, Kozlowska B, Wyzgal J, Soluch L, Walewska-Zielecka B, Rowinski W, Lao M: Long-term results of treatment of chronic hepatitis B, C and D with interferon-alpha in renal allograft recipients. Transpl Int 11 Suppl 1:S135-S139, 1998
16. Baid S, Tolkoff-Rubin N, Saidman S, Chung R, Williams WW, Auchincloss H, Colvin RB, Delmonico FL, Cosimi AB, Pascual M: Acute humoral rejection in hepatitis C-infected renal transplant recipients receiving antiviral therapy. Am J Transplant 3:74-78, 2003
17. Ichikawa Y, Kyo M, Hanafusa T, Kohro T, Kishikawa H, Fukunishi T, Nagano S, Shinji Y: A 20-year case study of a kidney transplant recipient with chronic active hepatitis C: clinical course and successful treatment for late acute rejection induced by interferon therapy. Transplantation 65:134-138, 1998
18. Tang S, Cheng IK, Leung VK, Kuok UI, Tang AW, Wing HY, Neng LK, Mao CT: Successful treatment of hepatitis C after kidney transplantation with combined interferon alpha-2b and ribavirin. J Hepatol 39:875-878, 2003
19. Eardley KS, Jones HE, Osman H, Smith SA: Efficacy of the accelerated hepatitis B vaccination schedule used in haemodialysis patients postexposure to virus: a single centre experience. Nephrol Dial Transplant 17:1982-1987, 2002
20. Dando T, Plosker G: Adefovir Dipivoxil: A Review of its Use in Chronic Hepatitis B. Drugs 63:2215-2234, 2003
21. Fabrizi F, Lunghi G, Poordad FF, Martin P: Management of hepatitis B after renal transplantation: an update. J Nephrol 15:113-122, 2002
22. Kamar N, Sandres-Saune K, Selves J, Ribes D, Cointault O, Durand D, Izopet J, Rostaing L: Long-term ribavirin therapy in hepatitis C virus-positive renal transplant patients: Effects on renal function and liver histology. Am J Kidney Dis 42:184-192, 2003
23. Chang KM, Rehermann B, Chisari FV: Immunopathology of hepatitis C. Springer Semin Immunopathol 19:57-68, 1997
24. Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr., Haussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J: Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 347:975-982, 2002
25. Bruchfeld A, Stahle L, Andersson J, Schvarcz R: Interferon and ribavirin therapy in dialysis patients with chronic hepatitis C. Nephrol Dial Transplant 16:1729, 2001
26. Fabrizi F, Martin P, Ponticelli C: Hepatitis C virus infection and renal transplantation. Am J Kidney Dis 38:919-934, 2001
27. Kamar N, Toupance O, Buchler M, Sandres-Saune K, Izopet J, Durand D, Rostaing L: Evidence that clearance of hepatitis C virus RNA after alpha-interferon therapy in dialysis patients is sustained after renal transplantation. J Am Soc Nephrol 14:2092-2098, 2003
28. Cruzado JM, Casanovas-Taltavull T, Torras J, Baliellas C, Gil-Vernet S, Grinyo JM: Pretransplant Interferon Prevents Hepatitis C Virus-Associated Glomerulonephritis in Renal Allografts by HCV-RNA Clearance. Am J Transplant 3:357-360, 2003
REFERENCES
Correspondence to:
Thomas Fehr M.D., Transplantation Biology Research Center,
Massachusetts General Hospital,
MGH East CNY 149, 13th street,
Boston, Massachusetts 02129, USA;
Phone: +1 617 726 4070, Fax: +1 617 724 9892,
E-mail: thomas.fehr@tbrc.mgh.harvard.edu