
|
Paneles de Discussión
Paneais de Discussio |
Franco Ferrario Renal Immunopathology Center – S. Carlo Borromeo Hospital Milan, Italy INTRODUCTION IgA nephropathy is the most common histological type of glomerulonephritis [1]. The glomerular histopathology of IgAN is commonly classified as mesangial proliferative GN with a large spectrum of mesangial involvement, ranging from minimal to diffuse proliferation [2]. Segmental necrotizing glomerular lesions usually with concomitant extracapillary proliferation, now universally considered to be the marker of a vasculitic damage of the glomerular capillary wall, are a rather frequent finding in the acute flareups of Henoch–Schönlein purpura, but are considered a very rare finding in idiopathic IgA nephropathy . Their presence in a small number of patients with this disease had been reported in some older studies on biopsy material [3–4]. However, none of the subsequent numerous studies on large series of patients, have focused attention on the presence of these necrotizing lesions and on their prognostic role in idiopathic IgAN [5, 6]. All of these classifications of the morphologic glomerular lesions that have been used to predict the prognosis were founded either on the separate analysis of single histologic features that did not include segmental necrosis of the capillary loops, or on a grading system based on the extent and severity of the overall renal lesions, usually derived from that proposed in 1982 by Lee et al, in which glomerular necrosis was not considered among the characterizing features. Extracapillary proliferative lesions, usually present as nonwidespread noncircumferential crescents frequently associated with glomerulocapsular adhesion, have been reported in all of the previously cited studies in variable percentages of cases, and in few patients a more typical crescentic glomerulonephritis, with almost generalized involvement of all glomeruli, has been described. However, even when extracapillary proliferation was consistent, its presence was never considered a possible marker of intracapillary vasculitis, despite the established evidence of the relationship between capillaritis and crescents in both antineutrophil cytoplasmic antibody (ANCA)–positive vasculitis and Henoch–Schönlein purpura. More recently our group brought attention to the possible role of segmental glomerular necrosis as a variant of the disease[7]. In Japan, Shouno et al reported that by increasing the number of serial sections examined for any single biopsy specimen from the usual 20 to 100 in 128 patients with IgAN, the incidence of the segmental necrotizing lesion increased from 7% (wich is similar to that reported by previous investigators) to as much as 30%, suggesting that this lesion is probably more frequent than previously believed [8].
Morfological features The histological and immunohistological features of necrotizing–crescentic IgA nephropathy show a strict similarity with the necrotizing capillaritic lesion described in Henoch–Schönlein purpura [9] and in ANCA–associated vasculitis [10,11].
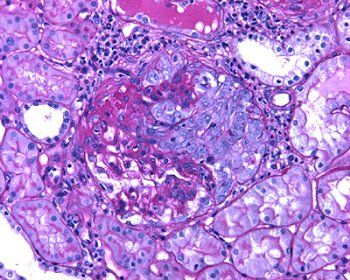
Fig. 2 – It is frequent the presence of extracapillary proliferation adjacent to the area of necrosis. 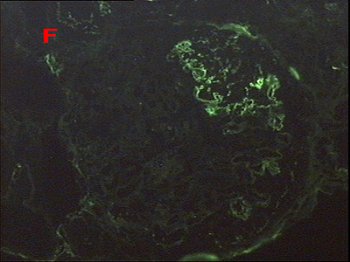
Fig. 3 – By immunofluorescence fibrinogen is strongly positive only in well delineated areas of the glomerular tuft, corresponding to areas of segmental intracapillary necrosis seen by light microscopy.
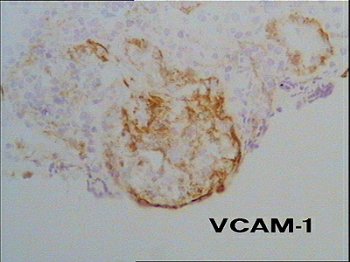
Fig. 6 – In the segmental area of necrotizing–extracapillary lesion is also present an abnormal expression of the adhesion molecule VCAM–1 that we already described as a distinctive marker of monocyte recruitment and adhesion in ANCA–positive vasculitis. 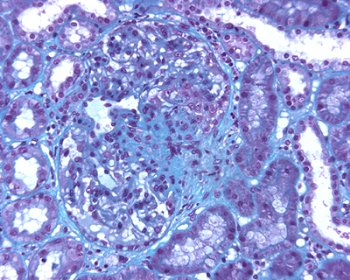
Fig. 7 – It is now well recognized that intraglomerular activated monocyte–macrophages are able to produce a great number of proinflamatory products that play an important role in the chronic sclerotic progression of renal damage. In our experience of repeat biopsies a well delineated segmental areas of glomerular scerosis with Bowman’s capsule adhesion were present, as a consequence of previous localized necrotizing–extracapillary lesions with monocyte accumulation. Personal experience We reviewed clinico–pathological features of our IgA nephropathy that met the following criteria: 1) light microscopy available containing a minimum of 10 glomeruli; 2) Immunofluorescence showing the prevalent deposition of IgA; 3) No signs of systemic involvement. Two hundred and sixty four patients met all of these criteria. Of the 264 cases, 35 (13,2%) showed the presence, at least in one glomerulus, of ncrotizing–extracapillary lesions. We compared the clinico–morphological features of these two groups of patients with necrotizing (35) and not necrotizing (Pure mesangial) IgA nephropathy (229).
a % of glomeruli (mean and range) b % of biopsies
The necrotizing lesions involved a variable percentage of glomeruli ranging from 3 to 25% (mean 8.6), and were usually associated with adjacent segmental areas of extracapillary cellular proliferation and also frequently with adhesions of the glomerular tuft to Bowman’s capsule. As indicated in Table 1, this extracapillary proliferation involved a percentage of glomeruli ranging between 8 and 53% (mean 24.5), significantly more marked than in the control population with IgA and absence of glomerular necrotizing lesions (mean 4.5). In this latter population, extracapillary proliferation that was frequently fibrocellular or fibrotic was present in 29% of the 229 patients, and in only 13 of them it involved more than 10% of glomeruli (10 to 37%). Another characterizing feature by light microscopy was the presence of interstitial infiltration of diffuse and/or segmental leukocytes, frequently with a periglomerular accentuation. As shown in Table 1, both focal and diffuse interstitial infiltration were found in a significantly higher number of biopsy specimens in comparison with the control population of patients with IgAN and absence of necrotizing lesions, while the extent of glomerular sclerosis and interstitial fibrosis did not differ in the two groups of patients. By IF, there was no difference in the frequency and intensity of deposition of IgA and IgG, and IgM, nor in the incidence of the combined mesangial and capillary staining for IgA between patients with and without necrotizing lesion. However, segmental areas of fibrinogen deposition were present in only the subgroup of patients with necrotizing lesions.
Clinical features
Table 2 compares demographic and clinical parameters at the time of biopsy in the 35 patients with necrotizing lesions and in 229 patients without necrotizing lesions selected as previously indicated. Both groups had a similar age and male predominance. Mean serum creatinine levels were not significantly different. Gross hematuria at the time of biopsy or a previous history of recurrent macroscopic hematuria were reported in similar percentages of patients. Frequency of arterial hypertension was not significantly different in the two groups. The mean duration of the prebiopsy follow–up was longer in patients without signs of necrotizing vasculitis than in patients with necrotizing lesions: 60 months (range 3 to 240) versus 33 months (range 4 to 191). Only the average amount of urinary daily loss of proteins at the time of biopsy was significantly different in the two groups of patients: It was higher in patients with segmental necrotic lesions. Although we searched for IgG ANCAs in 22 of the 35 patients with necrotizing lesions using IF and ELISA, no patient had them.
Natural history In the last 10 year we started to treat all patients with necrotizing–crescentic IgA nephropathy. The treatment was identical to that in use in our unit for the patients with rapidly progressive crescentic glomerulonephritis caused by vasculitis. It consisted of steroids (3 intravenous pulses of 0.75 to 1.0 g of methylprednisolone, followed by oral prednisone for 6 months at progressively tapering doses, starting with 0.5 mg/kg/day) and cyclophosphamide (2 mg/kg/day for 16 weeks). It was started in all patients immediately after biopsy. We compared the outcome of these 15 treated patients with the other 20 cases of ncrotizing–form of IgA nephropathy previously seen in our department and not treated. The extent of necrotic involvement and of extracapillary proliferation was comparable in the groups of treated and untreated patients (9 vs. 8.3% of glomeruli and 28.7 vs. 21.4% of glomeruli, respectively), and the difference in initial serum creatinine levels was not significantly different. Since immunosuppressive treatment was institued in the more recent years, the duration of follow–up was shorter in the 15 treated patients (mean 50.4 months; range 12 to 108 months) than in the 20 untreated patients (mean 74 months; range 12 to 204 months). During this period, only one of the treated patients progressed to end–stage renal failure (ESRF) requiring dialysis, this occurring 48 months after biopsy. By comparison, 7 of the 20 patients who did not receive immunosuppressive treatment progressed to ESRF, with a mean interval from the renal bioppsy of 70.2 months (range 36 to 96 months). A Kaplan–Meier estimate of renal survival in the 229 patients without necrotizing lesions selected as the control group was compared with that of the subgroup of 15 patients with necrotizing IgAN who received treatment with steroids and cyclophosphamide and of the subgroup of 20 patients who did not receive treatment (Table 3). Despite the trend to a more rapid loss of renal function in untreated patients with necrotizing IgAN, the difference in the rate of progression did not reach statistical significance (P = 0.07).  DISCUSSION While segmental necrosis of the glomerular tuft usually asociated with extracapillary proliferation has always been considered a characterizing histologic lesion in the glomerulonephritis of Henoch–Schönlein purpura, the less frequently documented presence of such a lesion even in some patients with idiopathic IgAN has received much less attention in the last 20 years, after its accurate description in many of the oldest morphological studies on this disease [3, 4 ]. The histological and immunohistological features of our 35 patients show a strict similarity with the necrotizing capillaritic lesion described in Henoch–Schönlein purpura [9] and in ANCA–positive systemic vasculitis [10–12]. Characteristics found in this disease were (1) a variable degree of extracapillary proliferation, usually segmental and adjacent to the areas of necrosis; (2) the intraglomerular influx of infiltrating cells, mainly monocytes, restricted to the areas of necrosis; (3) a significantly more marked interstitial accumulation of infiltrating cells (both monocytes and T lymphocytes) than in non–necrotic IgAN, with a frequent periglomerular accentuation; and (4) the intense deposition of fibrinogen in areas of tuft necrosis seen by IF. In the segmental areas of necrotizing and extracapillary lesions, we also found an abnormal expression of the adhesion molecule VCAM–1 that we had already described as a distinctive marker of the glomerular necrotizing lesions in ANCA–positive systemic vasculitis [11]. It is important to note that the extracapillary proliferation, although on average more marked than in the control cohort of patients without active signs of necrosis (Table 1), was not widespread and circumferential in our patients with necrotizing lesions; rather, the extracapillary proliferation was characteristically segmental and located in close vicinity to the areas of necrosis in the glomeruli. Frequently, it did not involve more than 10 to 15% of glomeruli and slightly exceeded half of them in only one patient. In other words, necrotizing lesions in idiopathic IgAN are not always associated with massive crescent formation, and noncircumferential extracapillary proliferation was sometimes the only sign of an extracapillary epithelial damage. We hypothesize that in a few patients with idiopathic IgAN and in a larger number with Henoch–Schönlein purpura, IgA immune complexed of a different size and/or affinity for the endothelium of the peripheral capillary wall may form and deposit from time to time in the glomeruli, to give the necrotizing, renal–limited vasculitic lesions described in the current study. Rebiopsies show that these lesions develop during the course of the disease and that, once developed, may heal, suggesting that they may represent an acute reversible phenomenon. The clinical features at the time of renal biopsy in the 35 patients of necrotizing–extracapillary lesions are similar to control group of 229 cases without these lesions, except for a statistically signficant higher degree of proteinuria, probably expression of more intense glomerular and interstitial inflammatory process. The untreated patients with necrotizing–extracapillary IgAN showed a tendency to progression to ESRF more rapidly and frequently than patients without necrotizing–extracapillary IgAN but also treated patients with necrotizing lesions. Also if this difference of the rate of progression did not reach statistical significante (P: 0.07), our data suggest that therapy with steroid and cyclophoshamide could provide good results in necrotizing–extracapillary IgAN.
REFERENCES
|