|
Discussion Board
Paneles de Discussión
Paneais de Discussio
Free Papers
Comunicaciones libres
Comunicaçoes livres
Home cin2003
Volver al Inicio cin2003
Voltar ao inicio cin2003
|
CLINICAL APPROACH TO THE PATIENT WITH A LARGE URINE OUTPUT:
Towards a better definition of polyuria
Andre F. Charest MD, Mathieu Lemaire MSc, Mohammad Ali Shafiee MD, Kamel S. Kamel MD, and Mitchell L Halperin MD
Division of Nephrology, St. Michael’s Hospital, University of Toronto,
Toronto, Ontario, Canada
mitchell.halperin@utoronto.caPolyuria is said to be present in adults on a typical Western diet when the urine volume is too large—typically > 3 L/day (Table 1). One reason to explain why 3 L/day was selected for this definition is that this volume is greater than the urine volume of 95% of otherwise normal adults. Another rationale for this > 3 L/day value is that normal adults excrete 600-900 milliosmoles per day. If their urine osmolality (U osm) was equal to their plasma osmolality (P osm), the urine volume would be as high as 3 L/day (dictated by dietary intake) (equation 1). This rationale does not include the fact that the kidney readily excretes urine with a higher U osm when vasopressin acts. In fact, normal subjects have a U osm > P osm throughout the 24-h cycle ( Figure 1).
Urine volume = # milliosmolesurine/Uosm (1)
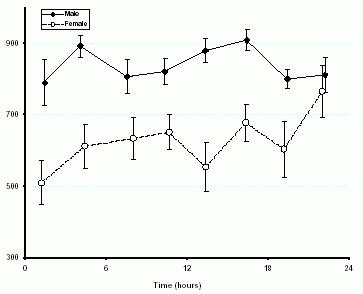
Figure 1.- Diurnal variation in the urine osmolality. Data were gathered in 75 adult volunteers. Urine was provided q2-h for the 16-h while awake plus an overnight sample. There were no restrictions on water intake, diet, or activity and there was no intake of medications.
Consultation: Why is this patient passing too much urine? The critical laboratory data to define the basis for this large urine volume are shown in Table 2. The additional clinical information confirms that this is polyuria because she drinks excessive volumes of bottled water, she runs several miles per day in a hot environment and she sweats profusely. She is trying to lose weight and is a vegetarian (10).
Almost every authority would agree that this patient has polyuria. Based on the Uosm of 80 mOsm/kg H2O, this is a water diuresis. Based on the plasma sodium (Na) concentration (PNa) of 130 mmol/l, it is due to excessive water intake. Important dangers for the patient are not obvious. The goals of therapy are clear—decrease her water intake to stop her polyuria.
Our approach: In this medical consultation, the reason for that consult should be defined as precisely as possible, threats to life should be identified, options for therapy outlined, expected responses predicted, and possible complications anticipated. To achieve these objectives, we strongly believe that principles of physiology should be applied at the bedside. The value of our approach is illustrated below.
(i) Physiology-based definition of polyuria: Compare the urine volume to expected values with the same provocative stimulus!!! In our terms, polyuria is present when the urine volume is higher than expected. As illustrated in appendix 1, there are times when polyuria could be present with a urine volume that is 0.5 L/day.
(ii) Discussion of the consult: Although this patient has primary polydipsia, we shall argue that she has a relatively low urine volume (not polyuria) because her 24-h urine volume is much less than it should be if vasopressin were absent due to her PNa of 130 mmol/l. When the urine flow rate is as high as possible, it will be >10 ml/min or 15 L/day in a patient with normal kidneys and fully suppressed vasopressin release. To evaluate the Uosm in a patient with a water diuresis, it is important to calculate the osmole excretion rate, especially. In this case, the osmole excretion rate is low, ~400 milliosmoles/day (5 L/day X 80 mOsm/kg H2O). With such a low osmole excretion rate (adults typically excrete 600-900 milliosmoles/day), her expected Uosm when vasopressin is maximally suppressed should be ~27 mOsm/kg H2O (400 milliosmoles/day/15 L/day) rather than the observed 80 mOsm/kg H2O.
(iii) What is the cause for this ‘lower’ urine volume of 5 L/day? One basis for her lower than expected urine volume could be a low, but not absent level of vasopressin released in response to non-osmotic stimuli. Because her expected Uosm is 27 instead of 80 mOsm/kg H2O signals a > 50% defect in ability to excrete electrolyte-free water—i.e., 5 instead of 15 L/day (equation 1).
There is also another possible diagnosis to explain her defect in excretion of water that led to the retention of ingested water and thereby hyponatremia. The low osmole excretion rate could be a signal to deduce that the volume of filtrate delivered to her distal nephron was considerably lower than the usual ~ 20 L/day. Now the combination of a low distal volume delivery and the presence of basal water permeability in the absence of detectable vasopressin (11) leads to what we have called, ‘trickle-down’ hyponatremia (12). This information is valuable at the bedside because we can now suggest dangers to anticipate and/or better options for therapy.
(a) Danger of acute hyponatremia: Because her urine volume was 5 and not 15 L/day, it was not the fact that the urine output was large, but rather that it was not large enough that placed her at risk. Therefore one can anticipate that she could retain more water and have an acute fall in her PNa if she ingested more water, had a non-osmotic stimulus for the release of vasopressin (13), and/or did not lose water in sweat because she did not exercise today. If any of the above occurred, she would be at risk of developing acute brain cell swelling and thereby herniation of the brain.
(b) Danger of osmotic demyelination: If she were to have a large intake of NaCl on one day, it is possible that this would increase the distal delivery of filtrate and thereby cause a large, acute water diuresis because there is no physiologic stimulus to release vasopressin (hyponatremia was present) (13). If overly-rapid correction of chronic hyponatremia took place, this patient might develop osmotic demyelination (14). This would be especially true if she had a more severe degree of chronic hyponatremia, was K deficient (15) and/or if her diet was nutritionally inadequate (16).
Concluding remarks: We prefer to use a physiology-based definition of polyuria (17) because it highlights the failure to excrete water rapidly enough despite a urine volume of 5 L/day. Moreover, potential dangers for the patient become easier to predict.
Appendix
1. Polyuria with a urine volume that is 0.5 L/day: When the PNa is in the high-normal range, water must be conserved so vasopressin will be released and cause the Uosm to be as high as possible. With a maximum Uosm of ~ 1000 mOsm/kg H2O in first-morning urines (Figure 1), the expected minimum urine volume in a patient on a low protein, low salt diet would be 0.25 L/day if that person excreted 250 milliosmoles/day (equation 1). In contrast, if this subject had an impaired renal concentrating ability with a maximum Uosm of 500 mOsm/kg H2O, the minimum urine volume would be 0.5 L/day when vasopressin acted. Therefore this 0.5 L/day urine output is twice the expected minimum value and indicates that there is polyuria due to an impaired renal concentrating ability. As discussed below, one should really analyze the urine volume in terms of its effective osmole content (18, 19).
2. Effective urine osmoles: In humans on a typical Western diet, close to half of the urine osmoles are urea. Because vasopressin causes the insertion of urea transporters into the luminal membrane of the inner medullary collecting duct (MCD)(20), the concentration of urea in its luminal fluid approaches that in the papillary interstitial compartment. Hence urea will not retain water in the inner MCD lumen. Therefore, urea is not an effective osmole in the inner MCD under these conditions and its excretion does not influence the urine volume (18).
Electrolytes, predominantly Na and Cl, account for the other half of the urine osmoles—they are effective urine osmoles. Therefore, it is more correct to say that the urine flow rate is proportional to the number of non-urea or effective urine osmoles and to their concentration in the medullary interstitial compartment when the distal nephron is permeable to water (equation 2) (19). There is a minor exception to this rule. Urea becomes a partially effective urine osmole when vasopressin acts if the rate of excretion of electrolytes is low, or if the excretion of urea is several-fold higher than usual (18, 21).
Urine flow rate = # (Effective osmoles)urine / [effective osmoles] urine (2)
REFERENCES
1. DeFronzo RA, Arieff AI. Disorders of sodium metabolism. In: Arieff AI, DeFronzo RA, editors. Fluid, Electrolyte, and Acid-Base Disorders. 2nd ed. New York, NY: Churchill Livingstone; 1995. p. 255-303.
2. Berl T, Robertson GL. Pathophysiology of water metabolism. In: Brenner BM, editor. Brenner & Rector's The Kidney. 6th ed. Philadelphia: W.B. Saunders Co; 2000. p. 866-924.
3. Bichet DG. Nephrogenic and central DI. In: Schreir RW, editor. Diseases of the Kidney. 6th ed. Boston, MA: Little Brown & Company; 1997. p. 2429-49.
4. Kokko JP. Approach to the patient with renal disease. In: Drazen JM, Gill GN, Griggs RC, editors. Cecil's Textbook of Medicine. 21st ed. Philadelphia, PA: W.B. Saunders Company; 2000. p. 526-32.
5. Bichet DG. Polyuria and diabetes mellitus. In: Seldin DW, Giebisch G, editors. The Kidney Physiology & Pathophysiology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2000. p. 1261-85.
6. DiGeorge A. Diabetes Insipidus. In: Behrman RE, editor. Nelson Textbook of Pediatrics. Philadelphia, PA: W.B. Saunders Company; 1996. p. 1574-6.
7. Burger HG, Loriaux DL, Marshall JC, Melmed S, Odell WD, Potts JT, Jr., et al. Vasopressin, DI and SIADH. In: DeGroot LJ, Jameson JL, editors. Endocrinology. Philadelphia, PA: W.B. Saunders Company; 2001. p. 363-76.
8. Denker BM. Polyuria. In: Brenner BM, editor. Harrison's Principles of Internal Medicinne. New York, NY: McGraw Hill; 2001. p. 267-8.
9. Richardson MS, Tobe S. Approach to the patient with polyuria or nocturia. In: Humes HD, editor. Kelly's Textbook of Internal Medicine. 4th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2000. p. 1108-12.
10. Thaler S, Teitlebaum I, Berl T. "Beer Potomania" in Non-Beer Drinkers: Effect of Low Dietary Solute Intake. Am J Kid Dis 1998;31:1028-31.
11. Halperin ML, Bichet DG, Oh MS. Integrative physiology of basal water permeability in the distal nephron: implications for the syndrome of inappropriate secretion of antidiuretic hormone. Clin Nephrol 2001;56:339-45.
12. Oh MS, Carroll HJ, Roy A, Denault N, Ledoux S, Remillard G, et al. Chronic hyponatremia in the absence of ADH: Possible role of decreased delivery of filtrate. J Am Soc Nephrol 1997;8:108A.
13. Robertson GL. Vasopressin. In: Seldin DW, Giebisch G, editors. The Kidney: Physiology & Pathophysiology. Philadelphia PA: Lippincott Williams & Wilkins; 2000. p. 1133-52.
14. Karp BI, Laureno R. Pontine and extrapontine myelinolysis: A neurologic disorder following rapid correction of hyponatremia. Medicine 1993;72:359-73.
15. Lohr JW. Osmotic demyelination syndrome following correction of hyponatremia: association with hypokalemia. Am J Med 1994;96:408-13.
16. Bahr M, Sommer N, Peterson D, Wietholter H, Dichgans J. Central pontine myelinolysis associated with low potassium levels in alcoholism. J Neurol 1990;237:275-6.
17. Halperin ML, Davids MR, Kamel KS. Interpretation of Urinary Electrolyte and Acid-Base Parameters. In: Brenner BM, editor. Brenner and Rector: The Kidney. 7th ed. Philadelphia: WB Saunders; 2003. p. In press.
18. Gowrishankar M, Lenga I, Cheung RY, Cheema-Dhadli S, Halperin ML. Minimum urine flow rate during water deprivation: Importance of the permeability of urea in the inner medulla. Kidney Int 1998;53:159-66.
19. Soroka SD, Chayaraks S, Cheema-Dhadli S, Myers JA, Rubin S, Sonnenberg H, et al. Minimum urine flow rate during water deprivation: importance of the urea and non-urea osmole concentration and excretion rate. J Am Soc Nephrol 1997;8:880-6.
20. Sands JM. The medullary collecting duct urea transporters. Current Opinions Nephrol Hypertens 1999;8:499-504.
21. Gamble JL, McKhann CF, Butler AM, Tuthill E. An economy of water in renal function referable to urea. Am J Physiol 1934;109:139-54.
Address all correspondence to:
Mitchell L. Halperin, MD, FRCP(C)
Professor of Medicine, University of Toronto
St. Michael's Hospital Annex, Lab #1, Research Wing. 38 Shuter Street
Toronto, Ontario, M5B 1A6, Canada
Email: mitchell.halperin@utoronto.ca
|
