
|
Paneles de Discussión
Paneais de Discussio |
ACYCLOVIR TREATMENT IN RENAL FAILURE PATIENTS Anders Helldén, M.D. Specialist in Nephrology, Resident in Clinical Pharmacology Div. of Clinical Pharmacology, Dept. of Laboratory Medicin, Karolinska Institutet, Huddinge University Hospital, Stockholm. Sweden INTRODUCTION Acyclovir is the drug of choice for treatment of herpes encephalitis caused by herpes simplex virus (HSV) or varicella zoster (VZV) infections, since the beginning of the 80:ths. Acyclovir dramatically improved morbidity and mortality in herpes encephalitis and is given to patients with suspected herpes encephalitis until the diagnosis has been excluded or as treatment. Valaciclovir (VACV), an acyclovir prodrug with improved bioavailability, is quickly metabolised to acyclovir and reaches 3-5 times higher plasma concentrations than oral acyclovir. Acyclovir has a reputation of being an atoxic drug with few side effects such as headache, nausea and gastrointestinal discomfort [1]. However, there is an increasing knowledge that acyclovir may cause severe adverse effects, such as CNS-symptoms and thrombotic microangiopathy, especially in patients with decreased renal function. A search in Medline in September 2003 on acyclovir and CNS-symptoms revealed at least 100 case reports. It has not been possible to connect high concentrations of acyclovir with acyclovir-toxicity, even if several reporters have suspected this in their reports [2, 3]. One main finding is that the majority of affected patients have had renal insufficiency. Interestingly there are some case reports of toxicity in patients without renal failure [4, 5]. Acyclovir is metabolized probably by alcohol dehydrogenase and aldehyde dehydrogenase to 9-carboxy-methoxymethylguanine (CMMG) and to a smaller extent to 8-hydroxy-9- (2-hydroxyethoxymethyl) guanine (8-OH-acyclovir) [6]. Intravenous acyclovir is excreted in the urine 60- 90 % unchanged and 1-10 % as CMMG in a patient without renal failure. The renal clearance for acyclovir is 100 - 300 mL/min indicating both glomerular filtration and tubular secretion [7]. The mean half-life of acyclovir is increased from approximately 2-4 hours in normal subjects to approximately 14-20 hours in patients with chronic renal failure. Renal insufficiency increases the urinary concentration of CMMG [8]. In these earlier studies on acyclovir metabolism the main metabolite CMMG was studied, but inconsistently. To our knowledge, there are no pharmacokinetics studies on CMMG in serum or plasma in renal patients. Acyclovir-induced neuropsychiatric symptoms in ESRD patients may develop within a few hours after the first dose [9]. Both intravenous administration and treatment with valaciclovir may yield high acyclovir concentrations, depending on the dose. Intravenous administration to previously healthy patients given too fast or without sufficient saline hydration may cause crystal precipitation in tubuli, resulting in acute renal failure [10] and decreased renal excretion of acyclovir. If acyclovir cannot be excreted, a greater part may be metabolized by hepatic metabolism to CMMG. Thus, it was hypothesised that increased serum concentrations of CMMG correlated to neuropsychiatric symptoms. If so, a simple blood test could support the diagnosis. PATIENTS AND METHODS The study was based on blood samples from 93 patients sent to the Department of Clinical Pharmacology, Huddinge University Hospital, Stockholm, Sweden for analysis of serum concentrations of acyclovir from November 1991 until June 1999. The patient's charts were reviewed to acquire information on e.g. age, sex and estimated creatinine clearance. Dosage and type of neuropsychiatric symptoms was also investigated. Furthermore, the result from clinical investigations such as EEG and CPR was analyzed. The research ethics committee of Huddinge University Hospital approved the study. Patients with encephalitis were also included, because they might have had symptoms both from their infection and acyclovir treatment. These patients were included in a mixed subgroup. RESULTS There were 49 patients with neuropsychiatric symptoms and 44 patients without symptoms. CMMG was increased in serum in patients with neuropsychiatric symptoms: 34.1 µmol/L (95% confidence interval 23.4 to 46.1) compared to a mean of 4.7 µmol/L, (95% confidence interval 3.3 to 6.6; P<0.001) for patients without symptoms. Acyclovir concentrations in the group with symptoms were 21.0 µmol/L ± 30.7 and in the group without symptoms 7.2 µmol/L ± 6.7 (P=0.004) [11]. The highest CMMG and acyclovir concentration in a single patient was 156 and 158 µmol/L, respectively. The sample was taken 3 days after treatment was instituted in a previously healthy patient with diabetes and acute renal failure due to intravenous acyclovir treatment.
CNS SYMPTOMS Tiredness, confusion and visual and auditory hallucinations were the most common symptoms reported among 37 patients, encephalitis patients excluded (table 1). The symptom tiredness is probably closely related to drowsiness or somnolence and might therefore have a higher magnitude than expressed here.  Table 1. The most frequent symptoms among 37 patients. ACYCLOVIR EXPOSURE To estimate ACV exposure in each patient an oral bioavailability (F) of 20 % for 200-mg doses, 13 % for 400-mg doses and 9 % for 800-mg doses, were assumed, based on earlier studies [12]. Valaciclovir was assumed to be 54 % bioavailable [13]. Exposure was calculated using the equation for steady state concentration (Css): Css = Here F is bioavailability, D is dose (in µmol), ACVCLn is the ACV clearance of an individual with normal renal function, CrCL is estimated creatinine clearance (in mL/min) and
CONCLUSION We found a strong correlation between increased CMMG concentrations and neuropsychiatric symptoms. Determination of CMMG in serum might facilitate to distinguish between CNS-symptoms caused by the encephalitis itself or by acyclovir treatment. CASE The usefulness of CMMG determination can be illustrated by a case from the intensive care unit at our hospital. A 69-year-old liver transplanted recipient with anuria, multiple infections, increasing liver values and intermittent mechanical ventilation, developed cutaneous herpes simplex. Intravenous acyclovir, 300 mg once daily, was instituted according to renal function and weight (the patient was on continuous replacement therapy: "PRISMA"). The neurological status was normal when acyclovir treatment was instituted, but she suffered from pain, due to the blisters and polyneuropathy of "critical illness type". Sadness developed suddenly the day after acyclovir was instituted and the patient had difficulties to stay awake. Day 2 she became depressed and irritated and her condition deteriorated. Due to air hunger and dyspnoea on day 3, there was a need for continuous mechanical ventilation. At this moment the patient could only move her fingers and toes and it was not possible to establish an adequate contact. Acyclovir was withdrawn the same day, due to suspicion of acyclovir toxicity, and the first acyclovir and CMMG sample was collected (Figure 3). Her status improved slowly thereafter. Seven days after the first acyclovir dose was instituted she was lucid, smiling and could move arms and legs. As can be seen in the diagram the improvement follows the decline in acyclovir and CMMG. It is important to note that the acyclovir concentrations were within or below values normally seen following these doses. Hence, an analysis of acyclovir solely would not have been sufficient to make the diagnosis of acyclovir intoxication. 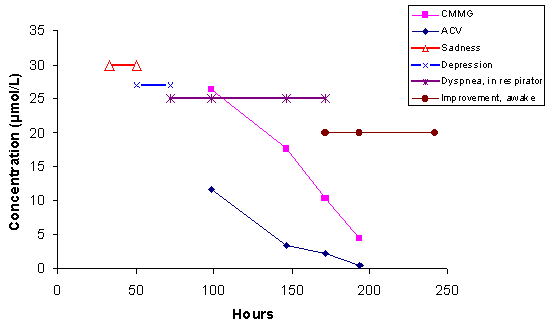 Figure 3. A case of acyclovir-induced neuropsychiatric symptoms from the ICU. DOSAGE AND TREATMENT OF ACYCLOVIR-INDUCED NEUROPSYCHIATRIC SYMPTOMS Firstly, adjust the dose to actual creatinine clearance. In our study we found that dose reduction was not performed in several patients. Adequate saline hydration is important when administrating intravenous acyclovir. There are published dose recommendations that take renal function into account [14]. Patients with ESRD are given 400 mg acyclovir as a starting dose and 200 mg twice daily. Patients with suspected CMMG intoxication and severe symptoms, such as unconsciousness or coma, improves by haemodialysis. A single haemodialysis session decreases the CMMG and acyclovir concentrations at least 50-60% (Figure 4) and often gives a dramatic clinical improvement [15]. It is important to take these measures, to prevent permanent damages. In patients with less severe symptoms acyclovir withdrawal and attempts to increase CMMG renal excretion is often enough, by hydration and forced diuresis.
Valaciclovir (500 mg once daily) can at present not be considered an option in ESRD patients due to the high exposure. Analysis of acyclovir and CMMG concentrations is recommended day two and five after oral acyclovir has been instituted, already after the first dose if intravenously administered or whenever there are signs of CNS-symptoms in an acyclovir-treated patient. REFERENCES [1]. Leflore S, Anderson P, Fletcher C. A risk-benefit evaluation of aciclovir for the treatment and prophylaxis of herpes simplex virus infections. Drug Saf 2000;23:131-142. [2]. Haefeli W, Schoenenberger R, Weiss P, Ritz R. Acyclovir-induced neurotoxicity: Concentration-side effect relationship in acyclovir overdose. Am J Med 1993;94:212-215. [3]. Feldman S, Rodman J, Gregory B. Excessive serum concentrations of acyclovir and neurotoxicity. J Infect Dis 1988;157:385-388. [4]. Vander T, Medvedovsky M, Herishanu Y. Encephalopathy induced by oral acyclovir in a patient with normal renal function. J Infect 2003;46(4):286. [5]. Braun J, Apel I, Schaffer S, Schumacher M, Berger M. Delirium during oral therapy of herpes zoster with acyclovir. Case report and brief review of central nervous system side-effects of acyclovir. Nervenarzt 1998;69:1015-1018. [6]. de Miranda P, Good S. Biotransformation of acyclovir to 9-carboxymethoxymethylguanine (abstract). Fed Proc 1982;41:1733. [7]. Blum RM, Liao SH, de Miranda P. Overview of acyclovir pharmacokinetic disposition in adults and children. Am J Med 1982;73(1A):186-192. [8]. de Miranda P, Whitley RJ, Blum MR, Keeney RE, Barton N, Cocchetto DM et al. Ayclovir kinetics after intravenous infusion. Clin Pharmacol Ther 1979;26:718-728. [9]. Bataille P, Devos P, Noel JL, Dautrevaux C, Lokiec F. Psychiatric side-effects with acyclovir. Lancet 1985;II:724. [10]. Perazella M. Crystal-induced acute renal failure. Am J Med 1999;106:459-465. [11]. Helldén A, Odar-Cederlöf I, Diener P, Barkholt L, Medin C, Svensson J et al. High serum concentrations of the acyclovir main metabolite 9-carboxymethoxymethylguanine in renal failure patients with acyclovir-related neuropsychiatric side effects: an observational study. Nephrol Dial Transplant 2003;18(6):1135-1141. [12]. de Miranda P, Blum MR. Pharmacokinetics of acyclovir after intravenous and oral administration. J Antimicrob Chemother 1983;12(SUPPL B):29-37. [13]. Blum MR, Soul-Lawton J, Smith CM, On NT, Posner,J, Rolan PE. Increased bioavalability of acyclovir from oral valaciclovir in healthy volunteers. Antiviral Res 1994;23(SUPPL. 1):74 Abstract. [14]. Almond MK. Avoiding acyclovir neurotoxicity in patients with chronic renal failure undergoing haemodialysis. Nephron 1995;69(4):428-432. [15]. Beales P, Almond MK, Kwan JT. Acyclovir neurotoxicity following oral therapy: Prevention and treatment in patients on haemodialysis. Nephron 1994;66:362-363. |