
|
Paneles de Discussión
Paneais de Discussio |
Mast cells in the kidney: what are they doing there?
Dr Ian Roberts Mast cells were first described by Paul Ehrlich in 1879. They were initially identified and sub-typed in tissue sections according to the dye-binding characteristics of their proteoglycan-rich granules1,2 (figure 1). Figure 1. Mast cells in the renal cortex stained with toluidine blue at pH 0.5, showing cytoplasmic granules
 In rodents, two distinct types of mast cells are present; mucosal and connective tissue mast cells, which differ in their staining characteristics, granule contents and functions. In humans, however, no such distinct mast cell populations can be defined by traditional histochemistry. The staining of mast cell granules with dyes such as toluidine blue shows variable sensitivity to tissue fixation and most mast cells fail to stain in formalin-fixed tissues. Up to 75% of renal mast cells are formalin-sensitive and cannot be detected by traditional histochemical stains in routine paraffin sections (table 1).
Diagnosis Mast cells/mm2 of cortex (median and range) p value Toluidine blue (pH 1.0) Mast cell tryptase Normal kidney 0.2 (0-0.8) 1.4 (0.3-3.6) 0.03 Chronic allograft rejection 12.5 (6.9-14.3) 41.3 (25.3-52.3) <0.01 Chronic pyelonephritis 7 (1.6-12) 37.8 (23.5-47.1) <0.01 More recently, immunohistochemistry (IH) for mast cell-specific enzymes tryptase and chymase has been used as a more reliable tool to identify and type human mast cells. The different functional roles of the three subsets so defined in humans (tryptase only positive, chymase only positive, tryptase and chymase positive) is currently unknown. Mast cell precursors originate from the bone marrow and circulate within peripheral blood, although at this stage they lack granules and are thus difficult to identify3, 4; the mature tissue mast cells are widespread, being present in virtually every organ in the body. The normal kidney, however, contains very few mast cells, which are mainly localised to the connective tissue around vascular bundles (figures 2 & 3). In contrast, mast cells can be seen to infiltrate the kidney in large numbers in many renal diseases (figure 4). An association between mast cells and renal disease was first noted by Pavone-Macaluso in 19605, and subsequently by Colvin and co-workers in 19746. After these reports, the link between mast cells and renal pathology was largely forgotten for over 20 years. In recent years, however, there has been renewed interest in the potential role of mast cells in the pathogenesis of renal failure and a number of reports have documented their presence in both primary renal disease and renal allografts. Figure 2.&3. Mast cells in the normal kidney: absent from the parenchyma, other than around vascular bundles
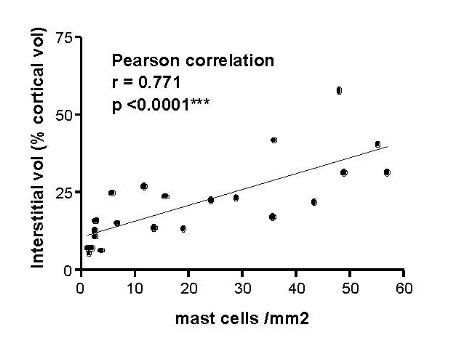
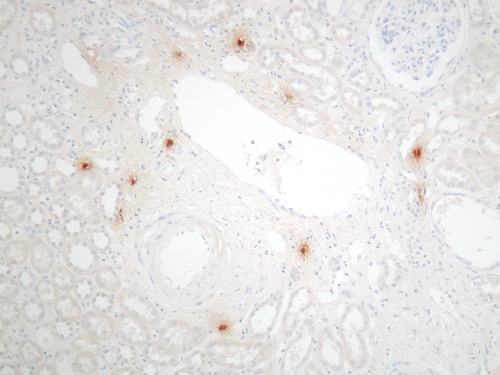 Mast cells are seen predominantly within the interstitium (figure 5); they also infiltrate tubules (figure 6), as described by Colvin. Epithelium of infiltrated tubules often shows tryptase positivity. Mast cells are not seen in normal arteries but are present in small numbers in the fibrotic intima of intra-renal arteries in chronic allograft vasculopathy (figure 7). They are very rarely seen in glomeruli. IH for tryptase commonly reveals evidence of mast cell degranulation, with tryptase positivity seen within the extracellular matrix around mast cells (figure 8). Mast cell infiltration is associated with interstitial fibrosis of multiple aetiologies7. Increased numbers are observed in diabetic nephropathy8, chronic glomerulonephritis7, 9, 10, 11 and chronic pyelonephritis7. In IgA nephropathy, there is a close correlation between the number of renal mast cells and extent of interstitial fibrosis (figure 9).
Figure 9. Correlation of mast cell infiltration and interstitial fibrosis in IgA nephropathy
 In renal allografts, mast cells are significantly increased in chronic allograft nephropathy, whether this is immune origin (chronic rejection) or due to chronic ciclosporin toxicity7, 12 (table 2). Table 2
Numbers of mast cells in renal allograft biopsies (reference 7)
At what stage of renal disease do mast cells appear and what are the triggers and mechanisms of recruitment? To answer the first question requires the longitudinal study of patients suffering an initial acute inflammatory insult, progressing later to fibrosis. Such evidence comes from renal transplantation. In patients biopsied during acute rejection and later, during chronic rejection, it is clear that mast cell infiltration is a late event, ie mast cells are associated with the development of fibrosis, not the initial immune response (table 2)7. Interaction between the growth factor receptor c-kit on the surface of mast cells and its ligand, stem cell factor (SCF), bound to fibroblasts appears to be central to recruitment and leads to mast cell chemotaxis, adhesion and activation. Interestingly, serum levels of SCF increase in patients with chronic renal disease13 and renal expression of SCF correlates with mast cell infiltration in many primary and secondary glomerular
diseases14. Many cytokines and other molecules, including PDGF, bFGF, VEGF, TNF and complement components, are chemotactic for mast cells but the most potent of all is TGF So what is the function of renal mast cells, and what is their link to renal pathophysiology? Evidence from diseases of organs other than the kidney indicates that the answer to this question may not be straightforward. Mast cells are best known for their role in allergic conditions, in which IgE-mediated degranulation results in the release of vasoactive amines. There is evidence that the production of vasoactive molecules, including histamine, leukotrienes and prostaglandins, by renal mast cells may modulate intra-renal haemodynamics. In addition, mast cells store, or are capable of synthesising, a wide variety of pro-inflammatory cytokines and enzymes and in view of the varied activity of mast cell products and their potential biological functions these cells have been implicated in many pathological situations. There is evidence that mast cells play a role in acute inflammation16, modulation of cellular immune responses17, angiogenesis18, turnover of connective tissue and fibrosis. It is the potential fibrogenic properties of mast cells that are of most interest in terms of their association with renal disease. Mast cells may potentiate fibrosis in a number of ways (recently reviewed by Gruber19). They are capable of synthesising several fibrogenic cytokines, including bFGF20 and TGF There is both in vitro and in vivo evidence that mast cells may play an important role in the development of pathological fibrosis. Co-culture of mast cells and fibroblasts results in fibroblastic proliferation that is modulated by direct cell-cell contact29. Mast cell granules are phagocytosed by fibroblasts in co-culture30 and ultrastructural studies have confirmed an intimate association between mast cells and fibroblasts in vivo (figure 10). Figure 10. Electron microscopy of a renal mast cell, showing close contact with interstitial fibroblasts
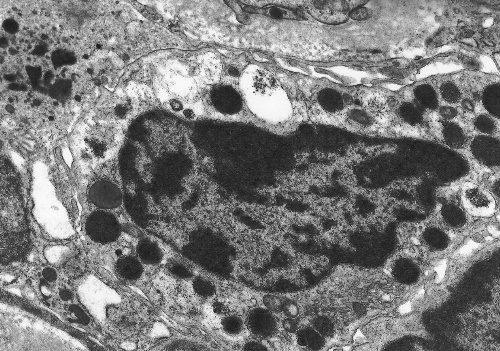 In addition to chronic renal disease, mast cells have been implicated in pathological fibrosis in a number of conditions including hypertrophic scars31, chronic atopic dermatitis32, hepatic cirrhosis33, fibrosing alveolitis34, 35 and cardiac fibrosis36. Studies using mast cell deficient mice (W/Wv) have demonstrated that these animals show less collagen deposition than their normal littermates in response to fibrogenic triggers, such as bleomycin-induced lung fibrosis37. Furthermore, cross breading of W/Wv mice with tight skin (TSK) mice, a strain that is genetically predisposed to developing dermal sclerosis, leads to a reduction in the level of fibrosis38. As yet, however, there is no data available using this strain of mice in a model of renal fibrosis. Renal mast cells should not be assumed to have pro-fibrotic activity without more direct in vivo evidence. In fact a recent study employing the PAN nephrosis model of chronic renal injury in a mast cell deficient rat strain (Ws/Ws) suggests that mast cells may have a protective rather than deleterious role in renal fibrosis39. Further studies are urgently required to clarify the function of renal mast cells. Mast cell stabilising agents have been demonstrated to be of benefit in inhibiting cutaneous fibrosis in an animal model40. It is possible that these compounds may provide a new approach to anti-fibrotic therapy in the management of chronic renal disease. References 1. Ehrlich P. Beitrage zur Kenntnis der granulierten Bindegewebszellen und der eosinophilen Leukocythen. Arch Anat Physiol (Physiol Abt), reprinted in Himmelweit F, ed. The Collected Papers of Paul Ehrlich Vol 1. Pergamon Press, London and New York, 1956:114-116. 2. Enerback L. Mast cell heterogeneity: the evolution of the concept of a specific mucosal mast cell. In: Beyfus AD, ed. Mast cell differentiation and heterogeneity. Raven Press, New York, 1986:1-26. 3. Horton MA, O'Brien HAW. Characterisation of human mast cells in long-term culture. Blood 1983;62:1251-1260. 4. Kirshenbaum AS, Goff JP, Dreskin SC, Irani AM,. Schwartz JB, Metcalfe DD. Interleukin 3-dependent growth of basophil-like and mast-like cells from human bone marrow. J Immunol 1982;142:2424-2429. 5. Pavone-Macaluso M. Tissue mast cells in renal diseases. Acta Pathol Microbiol Scand 1960;50:337-46. 6. Colvin RB, Dvorak AM, Dvorak HF. Mast cells in the cortical tubular epithelium and interstitium in human renal disease. Hum Pathol 1974;5:315-26. 7. Roberts ISD, Brenchley PEC. Mast cells: the forgotten cells of renal fibrosis. J Clin Pathol 2000;53:858-62. 8. Ruger BM, Hasan Q, Greenhill NS, Davis PF, Dunbar PR, Neale TJ. Mast cells and type VIII collagen in human diabetic nephropathy. Diabetologia 1996;39:1215-22. 9. Ehara T, Shigematsu H. Contribution of mast cells to the tubulointerstitial lesions in IgA nephritis. Kidney Int 1998;54:1675-83. 10. Kurusu A, Suzuki Y, Horikoshi S, Shirato I, Tomino Y. Relationship between mast cells in the tubulointerstitium and prognosis of patients with IgA nephropathy. Nephron 2001;89:391-97. 11. Hiromura K, Kurosawa M, Yano S, Naruse T. Tubulointerstitial mast cell infiltration in glomerulonephritis. Am J Kid Dis 1998;32:593-99. 12. Yamada M, Ueda M, Naruko T, et al. Mast cell chymase expression and mast cell phenotypes in human rejected kidneys. Kidney Int 2001;59:1374-81. 13. Kitoh T, Ishikawa H, Ishii T, Nakagawa S. Elevated SCF levels in the serum of patients with chronic renal failure. Br J Haematol 1998;102:1151-6. 14. El-Koraie AF, Baddour NM, Adam AG, et al. Role of stem cell factor and mast cells in the progression of chronic glomerulonephritides. Kidney Int 2001;60:167-72. 15. Jones SE, Kelly DJ, Cox AJ, et al. Mast cell infiltration and chemokine expression in progressive renal disease. Kidney Int 2003;64:906-13. 16. Wershil BK, Murakami I, Galli SJ. Mast cell dependent amplification of an immunologically non-specific inflammatory response. J Immunol 1988;140:2356-2360. 17. Gershon RK, Askenase PW, Gershon MD. Requirement for vasoactive amines for production of delayed type hypersensitivity skin reaction. J Exo Med 1975;142:732-747. 18. Meninger CJ, Zetter BR. Mast cells and angiogenesis. Semin Cancer Biol 1992;3:73-79. 19. Gruber BL. Mast cells in the pathogenesis of fibrosis. Current Rheumatology Reports 2003;5:147-153. 20. Qu Z, Liebler JM, Powers MR, Galey T, et al. Mast cells are a major source of basic fibroblast growth factor in chronic inflammation and cutaneous haemangioma. Am J Pathol 1995;147:564-573. 21. Hu ZQ, Yamazaki T, Cai Z, Yoshida T, Shimamura T. Mast cells display natural suppressor activity partially by releasing transforming growth factor-beta. Immunology 1994;82:482-486. 22. Johnson JL, Jackson CL, Angelini GD, George SJ. Activation of matrix-degrading metalloproteinases by mast cell proteases in atherosclerotic plaques. Arterioscler Thromb Vasc Biol 1998;18:1707-1715. 23. Cairns JA, Walls AF. Mast cell tryptase stimulates the synthesis of type I collagen in human lung fibroblasts. J Clin Invest 1997;99:1313-1321. 24. Gruber BL, Kew RR, Jelaska A, et al. Human mast cells activate fibroblasts: tryptase is a fibrogenic factor stimulating collagen messenger ribonucleic acid synthesis and fibroblast chemotaxis. J Immunol 1997;158:2310-7. 25. Kondo S, Kagami S, Kido H, Strutz F, Muller GA, Kuroda Y. Role of mast cell tryptase in renal interstitial fibrosis. J Am Soc Nephrol 2001;12:1668-76. 26. Taipale J, Lohi J, Saarinen J, Kovanen PT, Keskioja J. Human mast cell chymase and leukocyte elastase release latent transforming growth factor-b1 from the extracellular matrix of cultured human epithelial and endothelial cells. J Biol Chem 1995;270:4689-4696. 27. Ferrao AV, Mason RM. The effect of heparin on cell proliferation and type-I collagen synthesis by adult human dermal fibroblasts. Biochim Biophys Acta 1993;1180:225-230. 28. Norrby D. Effect of heparin, histamine and serotonin on the density-dependent inhibition of replication in two fibroblastic cell lines. Virchows Arch B Cell Pathol 1973;15:75-93. 29. Trautmann A, Krohne G, Brocker EB, Klein CE. Human mast cells augment fibroblast proliferation by heterotypic cell-cell adhesion and action of IL-4. J Immunol 1998;160:5053-5057. 30. Greenberg G, Burnstock G. A novel cell-to-cell interaction between mast cells and other cell types. Exp Cell Res 1983;147:1-13. 31. Smith CJ, Smith JC, Finn MC. The possible role of mast cells (allergy) in the production of keloid and hypertrophic scarring. J Burn Care Rehab 1987;8:126-131. 32. Leiferman KM, Ackerman SJ, Sampson HA, Haugen HS, Venencie PY, Gleich GJ. Dermal deposition of eosinophil granule major basic protein in atopic dermatitis. N Engl J Med 1985;313:282-285. 33. Armbrust T, Batusic D, Ringe B, Ramadori G. Mast cell distribution in human liver disease and experimental rat liver fibrosis. Indications for mast cell participation in development of liver fibrosis. J Hepatol 1991;26:1042-54. 34. Chanez P, Lacoste JY, Guillot B, Giron J, et al. Mast cells' contribution to the fibrosing alveolitis of the scleroderma lung. Am Rev Respir Dis 1993;147:1497-502. 35. Hunt LW, Colby TV, Weiler DA, Sur S, Butterfield JH. Immunofluorescent staining for mast cells in idiopathic pulmonary fibrosis: quantification and evidence for extracellular release of mast cell tryptase. Mayo Clin Proc 1992;67:941-948. 36. Turlington BS, Edwards WD. Quantitation of mast cells in 100 normal and 92 diseased human hearts. Implications for interpretation of endomyocardial biopsy specimens. Am J Cardiovasc Pathol 1988;2:151-157. 37. O'Brien-Ladner A, Wesselius LJ, Stechshulte DJ. Decreased lung collagen deposition in mast cell deficient mice after bleomycin. Am Rev Resp Dis 1990;42:A499. 38. Everett ET. The role of mast cells in the development of skin fibrosis in tight-skin mutant mice. Comp Biochem Physiol 1995;110:159-65. 39. Miyazawa S, Deenitchina SS, Hotta O, Natori Y. Protective role of mast cells on renal fibrosis: use of mast cell deficient rats. J Am Soc Nephrol 2001;12:711A. 40. Walker MA, Harley RA, Leroy EC. Inhibition of fibrosis in TSK mice by blocking mast cell degranulation. J Rheumatol 1987;14:299-301. | ||||||||||||||||||||||||||||||||||||||