
|
Paneles de Discussión
Paneais de Discussio |
Quality of Life in Patients with Chronic Renal Failure
Kamyar Kalantar-Zadeh, MD, MPH
Patients on maintenance hemodialysis (MHD) or chronic peritoneal dialysis (CPD) experience decreased QoL 6, 7 and significantly greater rates of protein-energy malnutrition and inflammation, together also known as malnutrition-inflammation complex syndrome (MICS) 8, 9. Moreover, dialysis patients have a worse QoL and higher rates of hospitalization and mortality compared with the normal population 10-14 Although the MICS has been correlated with both hospitalization and mortality rates, there are not many studies to examine whether this syndrome is associated with adverse QoL 2, 11, 15. The association between this somewhat subjective outcome, i.e. QoL, and other more objective measures, such as mortality and hospitalization, have been studied only recently in dialysis population.
The SF36, a short-form QoL scoring system with 36 items, is a self-administered questionnaire that was constructed to fill the gap between much more lengthy surveys and relatively coarse single-item measures of the QoL 2-5. Figure 1 shows the structure of SF36 scoring system. It consists of 36 questions, 35 of which are compressed into eight multi-item scales: (1) physical functioning is a ten-question scale that captures abilities to deal with the physical requirement of life, such as attending to personal needs, walking, and flexibility; (2) role-physical is a four-item scale that evaluates the extent to which physical capabilities limit activity; (3) bodily pain is a two-item scale that evaluates the perceived amount of pain experienced during the previous 4 wk and the extent to which that pain interfered with normal work activities; (4) general health is a five-item scale that evaluates general health in terms of personal perception; (5) vitality is a four-item scale that evaluates feelings of pep, energy, and fatigue; (6) social functioning (SF) is a two-item scale that evaluates the extent and amount of time, if any, that physical health or emotional problems interfered with family, friends, and other social interactions during the previous 4 wk; (7) role-emotional (RE) is a three-item scale that evaluates the extent, if any, to which emotional factors interfere with work or other activities; and (8) mental health is a five-item scale that evaluates feelings principally of anxiety and depression. Hence, in the SF36 scoring system, the scales are assessed quantitatively, each on the basis of answers to two to ten multiple choice questions, and a score between 0 and 100 is then calculated on the basis of well-defined guidelines, with a higher score indicating a better state of health 2. The scales of SF36 are summarized into two dimensions. The first five scales make up the "physical health" dimension, and the last five form the "mental health" dimension. The scales vitality and general health are parts of both dimensions (Figure 1). Hence, each dimension includes three specific and two overlapping scales 2. The SF36 also includes a question about self-evaluation of change in health during the past year (reported health) that does not belong to any score or dimension or the total SF36 score. The scores of the two dimensions and the total SF36 score are based on mathematical averaging of the scale components. Using Microsoft Excel 97, version 9.0 (Microsoft, Redmond, WA), we have designed a program based on well-defined SF36 guidelines to perform automatic scoring of the scales, dimensions, and the total SF36 results 2. Our reformatted SF36 questionnaire (English version) and the programmed Excel sheet to calculate the results of SF36 analysis along with related instructions as to how perform the questionnaire and its scoring are posted on the internet as an appendix to this article (www.nephrology.rei.edu/qol.htm ). The SF36 is a well-documented scoring system that has been widely used and validated as a QoL assessment tool for the general population as well as patients on MHD 3-5, 10, 16. It is used both as a stand-alone measure of QoL and as a core component of several major assessment tools, including the Kidney Disease Quality of Life (KDQOL) survey instrument 11, 17-19. The SF36 is one of the most commonly used instruments for QoL evaluation in patients undergoing maintenance dialysis. However, the true utility and applicability of SF36 for patients with ESRD have not been fully elucidated. Figure 1. The SF36 quality of life (QoL) scoring system and its scales and dimensions. Note that Vitality and General Health scales are overlapping components of both Physical Health and Mental Health dimensions. Question #2, self-evaluation of change in health during the past year (Reported Health), does not belong to any score, dimension or the total SF36 score 2.
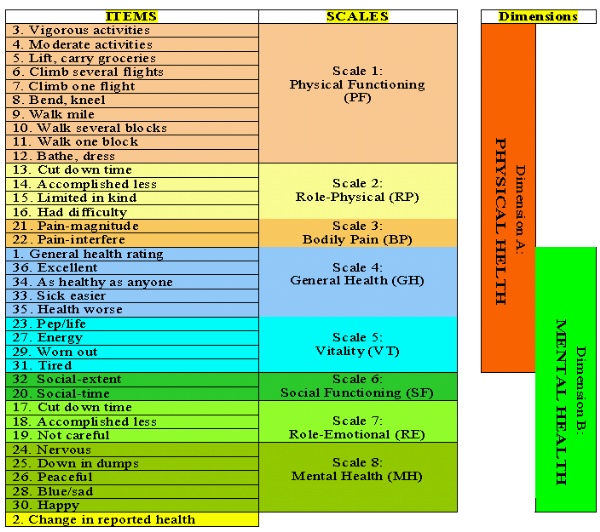
SF36 in Patients with Kidney Failure
Because the predialysis serum CRP showed a weak correlation with SF36, it is possible that at least part of the correlation between albumin, a visceral protein and an acute phase reactant, and the SF36 may be due to the fact that serum albumin is a marker of malnutrition-inflammation complex syndrome 2, an entity that may be associated with a worse QoL. SF36 as a Predictor of Mortality and Hospitalization in ESRD Patients
The Dialysis Outcomes and Practice Patterns Study (DOPPS) investigators analyzed their data from an international, prospective, observational study of randomly selected MHD patients in the USA (148 facilities), five European countries (101 facilities), and Japan (65 facilities) 11. The total sample size was composed of 17,236 patients. Using the KDQOL, they determined scores for: (1) physical component summary (PCS), (2) mental component summary (MCS), and (3) kidney disease component summary (KDCS). Complete responses on HRQOL measures were obtained from 10,030 patients. Cox models were used to assess associations between HRQOL and the risk of death and hospitalization, adjusted for multiple sociodemographic variables, comorbidities, and laboratory factors. For patients in the lowest quintile of PCS, the adjusted risk (RR) of death was 93% higher (RR = 1.93, P< 0.001) <>and the risk of hospitalization was 56% higher (RR = 1.56, P< 0.001) <>than it was for patients in the highest quintile level. The adjusted relative risk values of mortality per 10-point lower HRQOL score were 1.13 for MCS, 1.25 for PCS, and 1.11 for KDCS. The corresponding adjusted values for RR for first hospitalization were 1.06 for MCS, 1.15 for PCS, and 1.07 for KDCS. Each RR differed significantly from 1 (P< 0.001).<> For 1 g/dL lower serum albumin concentration, the RR of death adjusted for PCS, MCS, and KDCS and the other covariates was 1.17 (P< 0.01).<> They concluded that lower scores for the three major components of QOL were strongly associated with higher risk of death and hospitalization in MHD patients, independent of a series of demographic and comorbid factors. A 10-point lower PCS score was associated with higher elevation in the adjusted mortality risk, as was a 1 g/dL lower serum albumin level. More research is needed to assess whether interventions to improve quality of life lower these risks among hemodialysis patients 11. Finally, Lowrie et al 10 recently examined the data collected from 13,952 prevalent dialysis patients served by Fresenius Medical Care North America. Functioning and well-being were measured via the SF-36 Summary scale scores, PCS, and MCS. Also collected was information about hospitalizations and patient mortality. PCS and MCS were consistent predictors of hospitalizations and mortality rates even after adjustment for clinically relevant factors. They concluded that because PCS and MCS are associated with hospitalization and mortality, administering this self-report measure may serve as a valuable supplement to clinical measures traditionally relied on to predict patient outcomes. Moreover, such information may be unavailable through any other single mechanism 10.
It is imperative to examine all aspects of possible associations between such health survey questionnaires as the SF36 and clinically relevant indices such as nutritional state, inflammation and anemia and to explore the potentials of such scoring tools in predicting relevant clinical outcomes. The tool has to be a well-established and adequately validated one, both inclusive and user-friendly, with optimal capability of serving as an interviewer independent, self-administered questionnaire given the increasing time constraint involving health care personnel in charge of patients with ESRD. The SF36 may be a means to that end. Compared with those QoL tools that are tailored for patients undergoing dialysis, the SF36 has the advantage of being nonspecific, hence enabling the investigators to conveniently compare the health state of the patients with ESRD with non-ESRD populations under diverse observational and interventional studies.
1. Curtin RB, Lowrie EG, DeOreo PB: Self-reported functional status: an important predictor of health outcomes among end-stage renal disease patients. Adv Ren Replace Ther 6:133-140, 1999
2. Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH: Association among SF36 quality of life measures and nutrition, hospitalization, and mortality in hemodialysis. J Am Soc Nephrol 12:2797-2806, 2001
3. Maor Y, King M, Olmer L, Mozes B: A comparison of three measures: the time trade-off technique, global health-related quality of life and the SF-36 in dialysis patients. J Clin Epidemiol 54:565-570, 2001
4. Neto JF, Ferraz MB, Cendoroglo M, Draibe S, Yu L, Sesso R: Quality of life at the initiation of maintenance dialysis treatment--a comparison between the SF-36 and the KDQ questionnaires. Qual Life Res 9:101-107, 2000
5. Mingardi G, Cornalba L, Cortinovis E, Ruggiata R, Mosconi P, Apolone G: Health-related quality of life in dialysis patients. A report from an Italian study using the SF-36 Health Survey. DIA-QOL Group. Nephrol Dial Transplant 14:1503-1510., 1999
6. Chen YC, Hung KY, Kao TW, Tsai TJ, Chen WY: Relationship between dialysis adequacy and quality of life in long-term peritoneal dialysis patients. Perit Dial Int 20:534-540., 2000
7. Diaz-Buxo JA, Lowrie EG, Lew NL, Zhang H, Lazarus JM: Quality-of-life evaluation using Short Form 36: comparison in hemodialysis and peritoneal dialysis patients. Am J Kidney Dis 35:293-300., 2000
8. Kalantar-Zadeh K, Ikizler A, Block G, Avram M, Kopple J: Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis, November, 2003 [in press]
9. Kalantar-Zadeh K, Kopple JD: Relative contributions of nutrition and inflammation to clinical outcome in dialysis patients. Am J Kidney Dis 38:1343-1350, 2001
10. Lowrie EG, Curtin RB, Lepain N, Schatell D: Medical outcomes study short form-36: A consistent and powerful predictor of morbidity and mortality in dialysis patients. Am J Kidney Dis 41:1286-1292, 2003
11. Mapes DL, Lopes AA, Satayathum S, McCullough KP, Goodkin DA, Locatelli F, Fukuhara S, Young EW, Kurokawa K, Saito A, Bommer J, Wolfe RA, Held PJ, Port FK: Health-related quality of life as a predictor of mortality and hospitalization: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Kidney Int 64:339-349, 2003
12. Kalantar-Zadeh K KH, Supasyndh O, Kopple JD: Association between SF36 quality of life and serum albumin, creatinine and total lymphocyte count in hemodialysis patients. 11th International Congress on Nutrition and Metabolism in Renal Diseases, Nagoya, Japan; , March 2002 [abstract]
13. Kalantar-Zadeh K BG, McAllister CJ, Humphreys MH, Kopple JD: Self-reported subjective appetite correlates with inflammation, nutrition, and anemia and predicts quality of life and mortality in hemodialysis patients. ]. Annual conference of American Society of Nephrology, Am Soc Neph, November 2003 [abstract]
14. Kalantar-Zadeh K HM, Kopple JD: Association between type of dialysis access and quality of life in maintenance hemodialysis patients. J Am Soc Neph 13(Suppl):396A (abstract SA-P0649), Sep 2002 [abstract]
15. Isenring E, Bauer J, Capra S: The scored Patient-generated Subjective Global Assessment (PG-SGA) and its association with quality of life in ambulatory patients receiving radiotherapy. Eur J Clin Nutr 57:305-309, 2003
16. Martin MJ, Hulley SB, Browner WS, Kuller LH, Wentworth D: Serum cholesterol, blood pressure, and mortality: implications from a cohort of 361,662 m3n. Lancet 2:933-936, 1986
17. Bakewell AB, Higgins RM, Edmunds ME: Does ethnicity influence perceived quality of life of patients on dialysis and following renal transplant? Nephrol Dial Transplant 16:1395-1401., 2001
18. Kutner NG, Zhang R, McClellan WM: Patient-reported quality of life early in dialysis treatment: effects associated with usual exercise activity. Nephrol Nurs J 27:357-367; discussion 368, 424., 2000
19. Lo CY, Li L, Lo WK, Chan ML, So E, Tang S, Yuen MC, Cheng IK, Chan TM: Benefits of exercise training in patients on continuous ambulatory peritoneal dialysis. Am J Kidney Dis 32:1011-1018., 1998
20. Kalantar-Zadeh K, Block G, McAllister C, MH H, Kopple M: Appetite and inflammation, nutrition, anemia and clinical outcome in hemodialysis patients. [under review: Am J Clin Nutr]
21. Laws RA, Tapsell LC, Kelly J: Nutritional status and its relationship to quality of life in a sample of chronic hemodialysis patients. J Ren Nutr 10:139-147., 2000 October 2003 |