|
Discussion Board
Paneles de Discussión
Paneais de Discussio
Free Papers
Comunicaciones libres
Comunicaçoes livres
Home cin2003
Volver al Inicio cin2003
Voltar ao inicio cin2003
|
"INTERACTION OF BONE AND VASCULAR DISEASE IN CKD-5"
Paolo Raggi.
Professor of Medicine. Section of Cardiology.
Tulane University Health Sciences Center. New Orleans, LA, USA
praggi@tulane.edu
End stage renal disease is associated with hyperphosphatemia and hyperparathyroidism that often results in soft-tissue calcification. Indeed, arterial calcification is particularly common and associated in advanced renal failure stages (CKD-V).1-4 Recent studies have implicated the ingestion of calcium salts and/or the inevitable metabolic consequences of this therapy such as elevated serum calcium, hypercalcemic episodes, or suppression of parathyroid hormone as possible causative factors.3-7CKD patients are at high risk for cardiovascular disease and suffer from sudden death or myocardial infarction with much higher frequency than non-uremic individuals.
Though at entry in a dialysis program, most patients present a high burden of risk factors such as hyperlipidemia, diabetes mellitus, and hypertension, cardiovascular disease in CRF is explained by traditional risk factors only in part. 8
Indeed, recent evidence suggests that some of the risk may be associated with abnormalities of mineral metabolism such as hyperphosphatemia and an elevated calcium-phosphorus (Ca x P) product. 9. Though many patients with end-stage renal disease (ESRD) are in positive calcium balance, their bone tissue is paradoxically unable to handle the calcium load provided through calcium-based phosphate binders, dialysate and diet. Indeed, uremic osteodystrophy comprises 2 forms of bone disease: low turnover and high turnover bone disease. In the former state, bone is unable to handle calcium and there is minimal accretion of this mineral in the bone tissue. 10 In high turnover bone disease calcium is avidly absorbed by the bone but it is also rapidly removed. The end-result in either case is an excess availability of calcium in the circulation with the possibility to develop metastatic calcification of cardiovascular tissues as well as other soft tissues. Therefore, an effective control of serum phosphorus levels and Ca x P product appears to be very important to potentially reduce cardiovascular risk and improve bone health in CKD.
Pathogenesis of Cardiovascular Calcification in CKD
In patients affected by CKD-V the atherosclerotic plaques tend to be more extensive, more calcified and progress more rapidly (do we have a reference for this more rapid progression in ESRD?) than in age-matched non-uremic patients with known or suspected coronary artery disease. 11 Coronary, aortic and valvular calcification occurs at an early stage in CKD patients and even pediatric uremic subjects show extensive vascualar calcifications.
In 1988, Rostand and colleagues examined 43 dialysis patients using energy subtraction radiography to measure myocardial calcium content. They found that the dialysis patients had significantly higher myocardial calcium content than control subjects The process of vascular calcification is not entirely passive, as it is now believed to be an active process of mineral deposition resembling bone formation that can be initiated and promoted by hyperphosphatemia, hypercalcemia and hyperlipidemia. The combination of excess calcium load and elevated phosphorus levels in CKD patients causes a rise in the Ca x P product that may favor deposition of calcium in the soft tissues. It should be recognized that calcium in the atherosclerotic plaque accumulates mainly in the sub-intimal space and that CKD patients have an extensive plaque burden. However, extensive calcification of the medial layers of the coronary arteries and aorta are also possible and likely contribute to the extreme degree of calcification noted in this patient population.
The Investigative Tool
Electron beam computed tomography (EBCT) is a non-invasive radiological tool that can be employed to perform an accurate assessment of the presence and extent of calcification in the vasculature and cardiac valves. Quantification is performed by means of a scoring method that takes into consideration the size and density of a calcified focus. The calcification score measured by EBCT has been shown to correlate well with the occurrence of adverse cardiac events and silent myocardial ischemia in non-uremic subjects. 12 Similar data are also being accumulated in the CKD population. Finally, sequential EBCT scanning has been employed to evaluate the effectiveness of therapeutic strategies to slow the progression of coronary artery disease. 13-14
Sequential EBCT Studies in CKD 5 Patients
Chertow et al. recently evaluated 202 chronic hemodialysis patients using sequential EBCT imaging in the context of an international randomized clinical trial. Patients were randomized to treatment with one of 2 phosphate binders: a traditional calcium based salt or sevelamer (Renagel®) (Figure 1).15
At the end of the first 12 months of follow-up, the calcium treated patients showed a significant progression of the calcification score of the aorta (median increase 28%) and coronary arteries (median increase 25%), while the sevelamer (Renagel®) treated patients showed no change from baseline in either vascular territory (median increase 6% and 5% respectively, Figure 2 and 3).
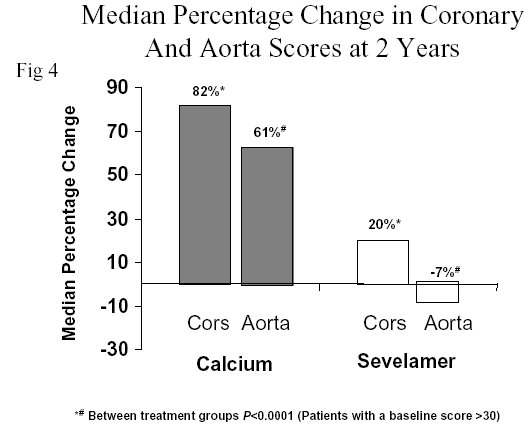
Seventy-three volunteers from the original cohort continued the study for 12 more months of randomization (Figure 1).At the end of 24 months of follow-up, the calcification progression in the aorta and coronary arteries had further magnified in patients treated with calcium salts reaching highly statistically significant values (82% and 61% increase, respectively; P<0.0001 from baseline). The patients treated with sevelamer (Renagel®) again showed no significant change from baseline (20% and –7%, respectively, Figure 4).
In view of the likely interaction of vascular and bone disease, in the extension trial the investigators also conducted an analysis of the change in bone density of the thoracic spine in the 2 treatment groups by means of CT densitometry. The patients treated with calcium salts showed a significant loss in trabecular bone density (-7%) and a non-significant decrease in cortical bone density (-2%). On the contrary, patients treated with sevelamer (Renagel®) showed a significant increase in trabecular bone density (+5%) and a non-statistically significant increase (+2%) in cortical bone density (Figure 5).
Conclusions
It has been suggested that inadequate management of calcium and phosphorus in CKD-V is an independent predictor of cardiovascular morbidity and mortality in this patient population. Both population based and CKD specific analyses point at an important interaction between bone and vascular health. Changess in management in calcium and phosphorus management directed to avoid directed at avoiding excess calcium load may reduce the risk of disease progression in both fields. Efforts to maintain Strict a strict control of the efforts to maintain serum phosphorus below 5.5 mg/dL, serum calcium below 9.5 mg/dl and Ca x P product below 55 mg2/dL2 have been recommended in an attempt to reduce the risks of uremic calcification, cardiovascular disease, and cardiac death. Sevelamer (Renagel®), a calcium-free phosphate binder, provides an important option for the management of dialysis patients at risk for cardiovascular calcification.
References:
- Braun J, Oldendorf M, Moshage W, et al. Electron beam computed tomography in the evaluation of cardiac calcification in chronic dialysis patients. Am J Kidney Dis 1996;27:394-401
- Guérin AP, Blacher J, Pannier B, et al. Impact of aortic stiffness attenuation on survival of patients in end- stage renal failure. Circulation 2001;103:987-992
- Oh J, Wunsch R, Turzer M, Bahner M, Raggi P, et al. Advanced coronary and carotid arteriopathy in young adults with childhood-onset chronic renal failure. Circulation 2002;106:100-105
- London G, Guerin AP, Marchais SJ et al. Arterial media calcification in end stage renal disease: impact on all cause and cardiovascular mortality. Nephrol Dial Transplant 2003;18:1731-40
- Goodman WG, Goldin J, Kuizon BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 2000;342:1478-1483
- Guérin AP, London GM, Marchais SJ, Metvier F. Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant 2000;15:1014-1021
- Raggi P, Boulay A, Chasan-Taber S, et al. Cardiac calcification in adult hemodialysis patients: a link between ESRD and cardiovascular disease? J Am Coll Cardiol 2002;39:695-701
- Longenecker JC, Coresh J, Powe NR et al. Traditional cardiovascular risk factors in dialysis patients compared with the general population: the CHOICE study. J Am Soc Nephrol 2002;13:1918-27
- Block, GA, Hulbert-Shearon, TE, Levin, NW, Port, FK Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 1998; 31: 607-17
- Kurz P, Monier-Fuagere MC, Bognar B et al. Evidence for abnormal calcium homeostasis in patients with adynamic bone disease. Kidney Int 1994;46:855-861
- Schwarz, U, Buzello, M, Ritz, E, Stein, G, Raabe, G, Wiest, G, Mall, G, Amann, K Morphology of coronary atherosclerotic lesions in patients with end-stage renal failure. Nephrol Dial Transplant 2000;15: 218-23
- Shaw L, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all cause mortality. Radiology 2003;228:826-833
- Callister, TQ, Raggi, P, Cooil, B, Lippolis, NJ, Russo, DJ Effect of HMG-CoA reductase inhibitors on coronary artery disease as assessed by electron-beam computed tomography. N Engl J Med 1998;339: 1972-8
- Raggi, P Regression of calcified coronary artery plaque assessed by electron beam computed tomography. Z Kardiol 2000;89: 135-9
- Chertow, GM, Burke, SK, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney International 2002;62:245-252
|
