|
Discussion Board
Paneles de Discussión
Paneais de Discussio
Free Papers
Comunicaciones libres
Comunicaçoes livres
Home cin2003
Volver al Inicio cin2003
Voltar ao inicio cin2003
|
ASSESSMENT OF HEALTH-RELATED QUALITY OF LIFE IN THE HEMO STUDY
Mark Unruh MD.
Assistant Professor of Medicine. University of Pittsburgh.
Renal-Electrolyte Division. Scaife Hall. Pittsburgh, PA. USA
UnruhM@msx.dept-med.pitt.edu
Patient reports of functioning and well-being or health-related quality of life (HRQOL) are recognized as providing important information about the impact of ESRD and its treatment on daily life[1-5]. HRQOL assessments may be used in patient care to screen for potential problems, to prioritize problems, to facilitate communication between health care workers and patients, and to monitor changes or response to treatment [6].
Generic HRQOL measures are designed to provide information about function and well-being that allows for comparison of individuals regardless of their specific condition. In contrast, disease-targeted measures collect information that is targeted towards the characteristics common to a subgroup of a population [7-9]. The most comprehensive assessment of HRQOL includes an assessment of both generic and disease-targeted content [10]. The Kidney Disease Quality of Life – Long Form (KDQOLTM-LF) was developed to provide a comprehensive assessment of health status for patients on hemodialysis. While HRQOL instruments may be administered by interview, by telephone, by self-administration, or by computer, the KDQOLTM-LF results have been reported as self-administered and interviewer-administered surveys [11].
Why does it matter how a survey is performed? The alternatives to self-administration of HRQOL instruments are particularly important in the ESRD population since these patients may be advanced in age and may have comorbid conditions that preclude traditional self-administration. We present KDQOLTM-LF results from individuals participating in the National Institute of Diabetes, Digestive and Kidney Diseases Hemodialysis (HEMO) Study, a randomized clinical trial designed to assess the effects of hemodialysis dose and membrane flux. The HEMO Study offered self-administered surveys or interviewer-administered surveys as an alternative for patients that were physically impaired or expressed a strong preference for interview-administration. This paper provides some complementary data to a previous study examining the baseline HEMO HRQOL results[11]. We examine the patient responses to dialysis symptoms and problems and then further examine extent to which mode of survey administration is related to HRQOL reports.
Study Design
The HEMO Study is a fifteen-center randomized clinical trial of the effects of hemodialysis dose and membrane flux on mortality and morbidity in patients undergoing chronic dialysis [12]. At randomization and annually, HEMO Study patients responded to a survey assessing health and satisfaction with care. Enrollment in the HEMO study began in March 1995 and ended December 2001. We report a cross-sectional analysis of responses to the KDQOLTM-LF at randomization from the first 1000 patients enrolled. Patient eligibility and data collection protocols have been previously described[13].
KDQOL-LF Assesses Generic and Kidney-Disease Targeted HRQOL Domains
The SF-36 is the generic core of the KDQOLTM-LF. The SF-36 has been extensively evaluated in the general population and the ESRD population [14, 15]. The SF-36 questions are grouped into 8 scales: Physical Functioning (10 items), Role-Physical (4 items), Bodily Pain (2 items), General Health (5 items), Vitality (4 items), Social Functioning (2 items), Role-Emotional (3 items), Mental Health (5 items) [16].
KDQOLTM-LF includes a Symptoms/Problems scale that assesses the extent to which symptoms bother the subject, such as dry itchy skin, thirst and hunger, pain in the joints or back, muscle cramps or soreness, and clotting or other problems with the access site. The Effects of Kidney Disease scale measures the impact of kidney failure on daily life with questions about restrictions on fluid and dietary intake, work, travel, lifting, and personal appearance. Other kidney-targeted domains measured by the KDQOLTM-LF have been previously described [7].
Data Analysis
Demographic and laboratory factors are described as means for continuous variables and frequency for dichotomous variables. The associations between scales with other continuous variables were examined using Pearson’s correlation coefficient. Differences between the self-administered and interviewer-administered groups were assessed using two sample t-statistics for interval or ratio-level variables (e.g. age) and Chi-square tests for categorical variables (e.g. race).
Two regression models were estimated for each KDQOLTM-LF scale to examine the associations of scale scores with survey mode of administration. The first model contained survey mode only and yielded the unadjusted difference between self-administered and interviewer-administered methods. We examined a second model that included the full set of covariates and yielded adjusted estimates of differences by mode of administration. Propensity scores were developed using logistic regression with self-administration as the outcome variable.
Reclassifying Mode of Administration Variable
In order to further look for differences among the interview-administered group, the mode of administration was reclassified into a three-level variable for mode of administration: self-administration, interview-administration due to physical impairment such as poor vision or manual dexterity, and interview-administration due to strong patient preference. The reclassified categorical variable was tested with multiple linear regression in a full set of covariates as described previously. The significance testing of the supplemental model was comparison of each of the interviewed-groups to the self-administration group.
A P value of less than or equal to 0.05 was considered statistically significant. All significance tests are two-tailed. All analyses were performed with SAS for Windows 6.12 [17].
Results
Of the first 1000 patients enrolled in the HEMO Study, 978 completed the KDQOLTM-LF questionnaire at baseline. The 22 patients who did not respond to the survey did not speak either English or Spanish. Table 1 shows relevant sociodemographic and clinical characteristics of the 978 respondents. The average age was 57.6 years and 53.7% were female. Almost two-thirds were African-American and 5% were Hispanic. A majority had diabetes or hypertension as the cause of ESRD and approximately one-third had severe comorbidity. The average albumin was 3.7 g/dl and average hematocrit was 33%.
Overall, 44% of study patients were interviewed and the remainder self-administered the KDQOLTM-LF. Of all those interviewed, 42% were interviewed because of poor vision, 43% because of patient preference, and 15% because of limited manual dexterity. As shown in Table 1, patients who responded to the interviewer-administered questionnaire were on average older, more likely to be female, African-American, to have diabetes as the cause of ESRD, and to have a high burden of comorbid disease. The interviewer-administered subjects had significantly lower mean serum albumin and creatinine, and a lower percentage of patients were listed for transplantation.
Responses to individual items
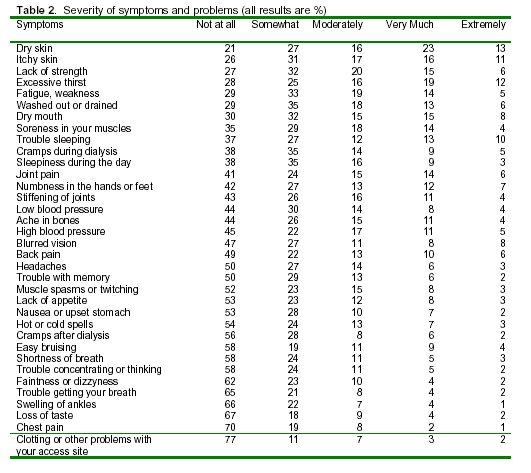
While scale scores allow analysis and group comparisons, responses to individual items may be of interest in clinical practice. As Table 2 demonstrates, many of the HEMO study subjects were bothered by particular symptoms. The symptoms are ranked in descending order according to the proportion of subjects who reported being bothered at all by a symptom. Thus, the first symptoms listed are those bothering the largest proportion of subjects at least "somewhat." The most common symptoms, bothering at least 60% of subjects, were dry skin, itchy skin, lack of strength, excessive thirst, fatigue and weakness, feeling washed out or drained, dry mouth, muscle soreness, trouble sleeping, cramps during dialysis, and sleepiness during the day. In contrast, more than 60% were not at all bothered by faintness or dizziness, trouble getting your breath, swelling of ankles, loss of taste, chest pain, and clotting or other problems with access site.
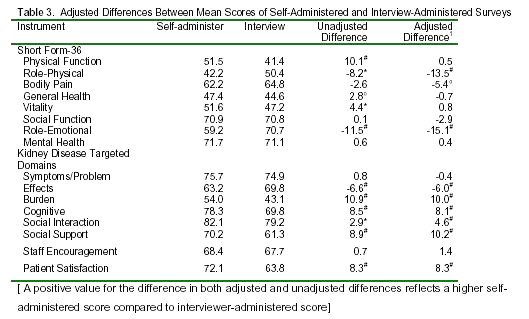
 As Table 3 shows, there are differences in SF-36 scores and kidney disease targeted domain scores between self-administered group and the interview-administered group. In unadjusted analysis (left column), the average scores for Physical Functioning, General Health, Vitality, Burden of Kidney Disease, Cognitive, Social Interaction, Social Support and Patient Satisfaction were substantially higher (better health) in the self-administered group. The average scores for Role-Physical, Role-Emotional and Effects of Kidney Disease were substantially lower (worse health) in the self-administered group. After adjusting for demographic and case mix variables, the differences in scores were substantially diminished in Physical Functioning, General Health, and Vitality, however, many differences remained. The scores measuring Role-Physical, Role-Emotional, Bodily Pain and Effects of Kidney Disease were significantly lower (worse functioning and well-being) in the self-administered group (all p<0.05). On the other hand, the scores for Patient Satisfaction, Social Support, Quality of Social Interaction, Cognition, and Burden of Kidney Disease were significantly higher (better health) in the self-administered group (all p<0.01) The R2 for the SF-36 domains ranged from 0.05 for mental health to 0.27 for Physical Functioning (all p <0.01), and the R2 for the kidney disease-targeted domains ranged from 0.04 for Dialysis Staff Encouragement and 0.11 for Burden of Kidney Disease (all p<0.01 except Dialysis Staff Encouragement p=0.059).
 The differences between mode of administration were consistent when examining interview-administered due to physical impairment and interview-administered due to patient preference as shown in Table 4. The scores for Patient Satisfaction, Social Support, Quality of Social Interaction, Cognition, and Burden of Kidney Disease were significantly higher (better functioning and well-being) in the self-administered survey group compared to both physical impairment and preference groups after adjustment for covariates (p<0.05). Also consistent with the findings presented above, the scores measuring Role-Physical, Role-Emotional, and Effects of Kidney Disease were significantly lower (worse health) in self-administered group compared to both the physical impairment and preference groups (p<0.05).
1 Multivariable model adjusted for age, race, sex, ICED comorbidity index, years on dialysis, serum albumin, normalized protein catabolic rate, serum creatinine, equilibrated Kt/V, diabetes, listed for transplantation, and study center. ° P<0.05, * P< 0.01, # P< 0.001
We further performed a propensity score predicting self-administration using predictors such as age, race, comorbidity, diabetes, and vintage. We then stratified the score by quintile and examined the relationship between the propensity score and HRQOL scale scores. There results are presented in Table 5. The results in this table support the selection bias from self-administered HRQOL surveys. In this table, we find that the association between the propensity score for self-administration and HRQOL scores were particularly strong in the domains of physical well being such as physical functioning, general health, and vitality. Furthermore, the association between the propensity score and HRQOL scores were also strong in burden of kidney disease and cognition, these areas also seem like they should be related to ability to self-administer a survey. We felt that these results were very complementary to work that we have previously published[11].
Discussion
Health-related quality of life has burgeoned as an outcome measure in patients with ESRD. Despite wide use, previous studies using HRQOL scores have overlooked the effects of survey administration on patient responses in the dialysis unit. In contrast to previous studies of patients with ESRD, this survey was conducted both by interview and self-administration, and the analysis accounts for mode of administration [14, 15, 19, 20]. Significant differences between interviewer administered and self-administered results persisted after controlling for sociodemographic and clinical features. These differences were similar for patients with interviewer-administered survey due to strong patient preference or physical disability. The association of mode of administration with HRQOL results suggests either that there are unmeasured differences between patients who are surveyed by self-administration compared to those who are surveyed by interviewer-administration, or that the mode of administration influences the results.
Other studies of ESRD patients have included health status results from both self-administration and interview-administration, but fail to report the number of patients interviewed or adjust for mode of administration . Combining patient scores from different modes of administration may confound and make difficult the interpretation of scores. In addition, some authors have made comparisons between scores in different studies without accounting for mode of administration. For example, one study in ESRD reports SF-36 scores on self-administered surveys from Italy, and compares these to previously published scores from patients in the United States and in England [21]. Since the scores from the United States reflected a mixture of self-administered and interviewer-administered surveys [14], any differences between the countries could reflect differences associated with survey administration.
Studies that have only included self-administered HRQOL, may miss many patients. As previously noted, patients on dialysis frequently have problems with vision and also difficulty with manual dexterity given that ones arm ranges is limited during hemodialysis cause difficulty with self-assessment [22]. Interviewers also offer a clear advantage for those respondents who have difficulty reading and writing. Finally, there are many people for whom an oral interview is easier than self-administration, such as those who lack good reading and writing skills, whose first language is not English, or have difficulty seeing [23]. Despite that potential error introduced by interviews, these were necessary to achieve a high rate of item completion were performed for over 40% of patients in the HEMO study.
Since the health status and satisfaction domain scores are valid and reliable, the HEMO health status baseline data allow comparison to other sample populations. Despite exclusion criteria, patients entering the HEMO Study report health status more similar to that of other prevalent dialysis patients than the general population or to a sample of patients with chronic renal insufficiency. In several domains, patients enrolled in the HEMO study report better health than patients in other dialysis populations [14, 15, 20]. In Figure 1, the HEMO study patients baseline SF-36 eight domain scores are appreciably higher than a large cross sectional survey of dialysis population [20]. The largest differences in scores among the dialysis studies are found in the SF-36 Role-Physical, Vitality, Social-Functioning, and Role-Emotional.
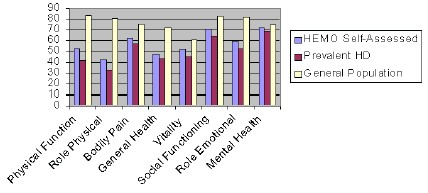
Figure 1. Comparison of HRQOL scores across the HEMO Study, Prevalent Dialysis and General PopulationWhile the scale scores of generic instruments allow for group comparisons, patient responses to kidney-disease specific items may also give providers improved familiarity with patient experience. The scores may guide clinicians when describing to patients what life is like on dialysis in the late 1990's. The kidney-disease specific scales suggest that well-dialyzed patients are not experiencing frequent chest pains, shortness of breath or edema.
On the other hand, Table 2 demonstrates that patients with ESRD commonly have symptoms associated with skin, thirst, and fatigue. While patients may provide this information on a self-assessment, they often will not voice these concerns to their physician unless prompted [22]. Disease specific measurement has the advantage of relating closely to areas of importance to clinicians [24]. These symptoms are often neglected although they have a major adverse effect on quality of life. The quantitative measurement of these symptoms may improve the patient's health status by leading clinicians to treat the problems thereby improving compliance with therapy and commitment to maintain functional status [3].
In conclusion, improvement in health-related quality of life in patients with ESRD is a major goal of the ESRD program in the US. Several interventions, including higher hematocrit, physical therapy, and nocturnal hemodialysis have been shown to improve HRQOL [5]. Thus, there is a need to measure HRQOL among patients supported by hemodialysis, including patients with advanced age and comorbidity, in whom self-administration may be more difficult.
The association of mode of administration with HRQOL results suggests either that there are unmeasured differences between patients who are surveyed by self-administration compared to those who are surveyed by interviewer-administration, or that the mode of administration influences the results. Investigators should include mode of survey administration in their reports. Providers need to interpret HRQOL results cautiously and consider how the information was gathered when using these instruments for patient care, quality control, or policy making. Finally, the development of new technology to measure HRQOL will be critical to including the patients most at risk in our assessments of quality of life.
Acknowledgments
First of all, I wish to thank the participants and investigators in the HEMO Study. In addition, I wish to thank Drs. Klemens Meyer and Andrew S. Levey for showing me the importance of HRQOL outcomes in ESRD and for their critiques of most of the material found in this paper. Furthermore, I gratefully acknowledge Guofen Yan MS, Milena Radeva MS, Ron D. Hays PhD, Robert Benz MD, John Kusek PhD for providing comments on various drafts of this paper. Lastly, I acknowledge Tom Greene, Ph.D. for his invaluable suggestions for data analysis.
References:
1. Rettig RA, Sadler JH: Measuring and improving the health status of end stage renal disease patients. Health Care Financing Review 18:77-82, 1997
2. Schrier RW, Burrowshudson S, Diamond L, Lundin AP, Michael M, Patrick DL, Peters TG, Powe NR, Roberts JS, Sadler JH, Siu AL, Lohr KN, Rettig RA: Measuring, Managing, and Improving Quality in the End-Stage Renal-Disease Treatment Setting - Committee Statement. American Journal of Kidney Diseases 24:383-388, 1994
3. Kutner NG: Assessing end-stage renal disease patients' functioning and well-being: measurement approaches and implications for clinical practice. [Review] [73 refs]. American Journal of Kidney Diseases 24:321-333, 1994
4. Kimmel PL: Just whose quality of life is it anyway? Controversies and consistencies in measurements of quality of life. Kidney International 57:S113-S120, 2000
5. Valderrabano F, Jofre R, Lopez-Gomez JM: Quality of life in end-stage renal disease patients. Am J Kidney Dis 38:443-464., 2001
6. Higginson IJ, Carr AJ: Measuring quality of life: Using quality of life measures in the clinical setting. Bmj 322:1297-1300., 2001
7. Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB: Development of the kidney disease quality of life (KDQOL) instrument. Quality of Life Research 3:329-338, 1994
8. Wu AW, Fink NE, Cagney KA, Bass EB, Rubin HR, Meyer KB, Sadler JH, Powe NR: Developing a Health-Related Quality-of-Life Measure for End-Stage Renal Disease: The CHOICE Health Experience Questionnaire. Am J Kidney Dis 37:11-21, 2001
9. Laupacis A, Wong C, Churchill D: The use of generic and specific quality-of-life measures in hemodialysis patients treated with erythropoietin. The Canadian Erythropoietin Study Group. Controlled Clinical Trials 12, 1991
10. Bass EB, Jenckes MW, Fink NE, Cagney KA, Wu AW, Sadler JH, Meyer KB, Levey AS, Powe NR: Use of focus groups to identify concerns about dialysis. Choice Study. Medical Decision Making 19:287-295, 1999
11. Unruh M, Yan G, Radeva M, Hays RD, Benz R, Athienites NV, Kusek J, Levey AS, Meyer KB: Bias in Assessment of Health-Related Quality of Life in a Hemodialysis Population: A Comparison of Self-Administered and Interviewer-Administered Surveys in the HEMO Study. J Am Soc Nephrol 14:2132-2141, 2003
12. Eknoyan G, Levey AS, Beck GJ, Agodoa LY, Daugirdas JT, Kusek JW, Levin NW, Schulman G: The hemodialysis (HEMO) study: rationale for selection of interventions. Seminars in Dialysis 9:24-33, 1996
13. Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, Allon M, Bailey J, Delmez JA, Depner TA, Dwyer JT, Levey AS, Levin NW, Milford E, Ornt DB, Rocco MV, Schulman G, Schwab SJ, Teehan BP, Toto R, Hemodialysis Study G: Effect of dialysis dose and membrane flux in maintenance hemodialysis.[comment]. New England Journal of Medicine. Online. 347:2010-2019, 2002
14. Meyer KB, Espindle DM, DeGiacomo JM, Jenuleson CS, Kurtin PS, Davies AR: Monitoring dialysis patients' health status. American Journal of Kidney Diseases 24:267-279, 1994
15. DeOreo PB: Hemodialysis patient-assessed functional health status predicts continued survival, hospitalization, and dialysis-attendance compliance. American Journal of Kidney Diseases 30:204-212, 1997
16. Ware J, Snow K, Kosinski M, Gandek B: SF-36 Health Survey Manual and Interpretation guide. Boston, The Health Institute, New England Medical Center, 1993
17. Inc SI: SAS/STAT User's Guide Version 6.0. Cary, NC, SAS Institute Inc., 1990
18. Unruh M, Yan G, Radeva M, Hays RD, Benz R, Athienites NV, Kusek J, Levey AS, Meyer KB, Group atHS: Bias in assessment of health-related quality of life in a hemodialysis population: A comparison of self-administered and interviewer-administered surveys in the HEMO study. J Am Soc Nephrol 14, 2003
19. Painter P, Carlson L, Carey S, Paul SM, Myll J: Physical functioning and health-related quality-of-life changes with exercise training in hemodialysis patients. American Journal of Kidney Diseases 35:482-492, 2000
20. Diaz-Buxo JA, Lowrie EG, Lew NL, Zhang HY, Lazarus JM: Quality-of-life evaluation using short form 36: Comparison in hemodialysis and peritoneal dialysis patients. American Journal of Kidney Diseases 35:293-300, 2000
21. Mingardi G, Cornalba L, Cortinovis E, Ruggiata R, Mosconi P, Apolone G: Health-related quality of life in dialysis patients. A report from an Italian study using the SF-36 Health Survey. DIA-QOL Group. Nephrology, Dialysis, Transplantation 14:1503-1510, 1999
22. Kurtin PS, Davies AR, Meyer KB, DeGiacomo JM, Kantz ME: Patient-based health status measures in outpatient dialysis. Early experiences in developing an outcomes assessment program. Medical Care 30, 1992
23. Fowler FJ: Data Collection Methods, in Qulaity of Life and Pharmacoecomics in Clinical Trials, edited by Spilker B, Philadelphia, Lippincott-Raven, 1996
24. Parfrey PS, Vavasour HM, Henry S, Bullock M, Gault MH: Clinical-Features and Severity of Nonspecific Symptoms in Dialysis Patients. Nephron 50:121-128, 1988
|
