
|
Paneles de Discussión
Paneais de Discussio |
HEPATITIS B VACCINATION IN RENAL FAILURE PATIENTSD. VLASSOPOULOS, MDDepartment of Medicine, Division of Nephrology
|
|
Uremic syndrome (inflammation, anemia, malnutrition, hyperparathyroidism)
Age (old) Sex (female) Body weight (overweight) Iron overload HCV + Diabetes Genetics (MHC: HLA DR3/DR7 presence, B18, 51-DRB1 0101-DR15 absence) Dialysis (method, efficacy, biocompatibility) |
These genetic findings could be explained by the presence of a dominant immune gene in MHC, responsible for normal response (helper T cells activation) and, when present in MHC extended haplotypes on both chromosomes, responsible for low or no response (suppressor T cells activation) to HB virus (HBV) (or vaccine antigens) [12].
HLA DR3 was found associated with high TNF-α production, suggesting that this histocompatibility marker may play a role in regulating immune suppressive genes controlling the response to HB (S) antigen. However, more vigorous stimulation produces anti-HB (S) antibodies arguing in favor of a multifactor origin of the genetic immune defect (table 2) [5].
Specific antibody production, after hepatitis B vaccination, is generated via B-cell activation by class II (CD4+ Th1-helper) and class I restricted T-cell (CD8+ CTL-cytotoxic T cells) responses. Insufficient B and T-cell responses lead to chronic liver disease in 5-15% of the general population and in 30% of HBV infected dialysis patients [2, 16].
Immune suppression is associated with uremia and dialysis (toxins, low biocompatibility dialysis material, anemia, iron overload, trace element depletion, vitamin deficiency, malnutrition, secondary hyperparathyroidism) and leads to high susceptibility to infections, life-threatening sepsis and reduced response to vaccination [2, 17-20]. The number of blood transfusions and the presence of a hepatitis C virus (HCV) infection or diabetes mellitus are considered additional factors for the decreased immune response [21-23]. Indeed, response to hepatitis B vaccination is reported to be very low, in patients with hepatitis C virus infection, which suggests a possible genetic basis for low responder status to both viruses (table 2) [5, 22-23].
Scanty information exists concerning the relationship between dialysis adequacy, immune function and antibody response to vaccinations. There is, however, indirect evidence that more frequent dialysis may lead to an enhanced response because dialysis helps to restore impaired B7-2 expression [4]. In a study of peritoneal dialysis patients immunized with the hepatitis B vaccine, the initial weekly Kt/V was 2.37 and 2.01 in responders and non-responders respectively, although other investigators could not confirm such a favorable action of dialysis on immune function [5, 14, 19].
As a result of depressed immunity, dialysis patients are not able to respond to HB vaccination and when they do respond they develop lower antibody titers and do not maintain adequate antibody levels over time, compared to a healthy population [5, 24-27]. Some authors suggest that antibody response to HB vaccination can be correlated to the degree of renal failure but not to the specific dialysis mode (peritoneal dialysis or haemodialysis) while others suggest that antibody response can be modulated by the type of dialysis used [20, 24].
Intradermal route of HB vaccination provides a stronger cellular and humoral stimulus than intramuscular injections, purportedly by recruiting relatively immature dermal dendritic Langerhans cells, which serve as antigen presenting cells [16].
There is less information concerning the response of ESRD and dialysis patients to tetanus, pneumococcus, diphtheria, influenza, varicella-zoster virus, and staphylococcus aureus vaccines [24-25, 28-32].
The antibody response to these vaccines in renal failure patients is also reported to be less than optimal [20, 32]. A prospective controlled study evaluating the response to tetanus and hepatitis B vaccines among patients with chronic renal failure, dialysis patients, transplant recipients, and healthy controls, found that antibody titers were lower in the groups with renal failure than in healthy vaccinated controls. Patients, who had previously responded to hepatitis B vaccine, were more likely to respond to tetanus vaccine, implying that a subset of patients have an intact immune system [25]. In other studies, however, tetanus and diphtheria vaccination responses have been found to be independent of responses to hepatitis B vaccination. This difference could be explained by the use of various vaccine preparation methods or by the presence of preexisting undetected antibodies to tetanus and diphtheria [24, 28].
The immune response in patients with ESRD is generally defective whenever T cell activation is required (hepatitis B, tetanus, influenza vaccine), while it is found almost normal when non-T dependent immunity mechanisms are activated (pneumococcal vaccine) [25]. Despite the evidence for decreased efficacy, current recommendations are to vaccinate patients with ESRD [20, 32].
Recipients of renal transplants respond to vaccines in a manner similar to chronic dialysis patients, as a result of immunosuppressive treatments such as cyclosporine administration. The antibody response is often less in these patients than in healthy controls and protective titers fall rapidly. Thus administration of vaccine is recommended, in susceptible patients, prior to transplantation. Post transplant vaccination or booster shots, have been safely applied in these patients [10, 17, 20, 25-26, 30-31, 33]. Live attenuated vaccines (Measles, Mumps, Rubella, Varicella) should be avoided in transplanted patients [20, 31].
III) HEPATITIS B INFECTION IN RENAL FAILURE AND DIALYSIS: DEFINITION AND MAGNITUDE OF THE PROBLEM
Hepatitis B infection in dialysis centers depends on the disease’s prevalence in general and particularly in the dialysis population. Hepatitis B has been on the decline in the last decade in renal units, a consequence of efficient prophylaxis measures. The most important factor in preventing the spread of hepatitis B in haemodialysis units has been the maintenance of universal precautions. CDC recommends isolating antigen-positive patients, treatment by a separate nursing team and prohibiting the use of shared medications (e.g., common heparin vials) in dialysis units [34-35]. High rates of exposure to hepatitis B virus were noticed only in renal units treating HB (S) Ag carriers. This exposure can be successfully limited by the above strict isolation procedures but despite this, accidents can spread the infection to the entire unit [1, 21, 34-37].
Hepatitis B vaccination in renal patients initially by a plasma derived vaccine containing attenuated live virus, although first reported 21 years ago as creating sufficient protection, has not been universally accepted as an efficient prophylaxis measure. Acceptance is limited mainly because of the unstable results in producing adequate response in all patients [10, 34, 36-42].
HB vaccination, when applied together with the other preventive measures, resulted in an up to 10fold drop in the number of new HB cases in haemodialysis patients and renal unit staff in Western Europe and in the United States [21, 34, 37, 43-44].
Although the incidence of the disease is actually very low, a high percentage of susceptible patients are still not vaccinated. Rules for the limitation of transmissible diseases in renal units give a sense of security to the staff, however, patient vaccination is still considered a secondary and costly procedure leading to a high percentage of unvaccinated patients. In USRDS report only 18.3% of adult patients were vaccinated against HBV in 2000. CDC suggests that the cost of vaccinating patients is mitigated by the reduced need for monthly surveillance of antigen and antibody status in those who develop specific antibodies [21, 34, 36-37, 39, 43, 45].
Hepatitis B is still a threat for dialysis patients, despite prophylactic measures, not only when they undergo dialysis in their home unit, but also when, with better patient rehabilitation, holiday traveling and dialysis in host centers is made possible. In these scenarios, the susceptible patient serves both as a potential HBV infection target (dialyzed in HBV positive centers) and as a transmitter of the disease back to the home unit [21, 35-36, 42, 46-47]. HBV could also infect dialysis patients by means common in the general population (sex etc). Infected patients can then spread the disease in their unit, before HB infection has been detected, if patients are not actively protected by a successful vaccination program [36].
A high percentage of dialysis patients becoming HBV positive will be unable to eliminate their virus (developing chronic hepatic disease). They are considered high risks for renal transplantation and are virus reservoirs to both other patients and non-protected staff [37]. A completely successful vaccination program is essential for patient and personnel protection against this chronic, persistent and potentially lethal disease [1, 21, 27, 34, 36, 40, 42, 44, 48].
Controversy exists concerning the overall effectiveness, including the cost/benefit ratio, of hepatitis B vaccination in patients with ESRD [10, 12, 14, 20, 22-23, 26-27, 34-43, 45, 47, 49-55].
Second generation recombinant vaccines (expressing the ‘S’ gene), have replaced plasma-derived vaccines (table 3). These vaccines are safer and result in immunogenicity levels similar to those seen in control patients [1, 45, 51].
| 1st Generation 1980 Plasma derived vaccines
2nd Generation 1988 Recombinant vaccines (pre-S: Engerix-Recombivax) 3rd Generation 2001 Recombinant vaccines (pre-S-S1-S2: Hepacare) |
Third generation recombinant vaccines containing pre-S1, pre-S2 and S antigenic components of both viral surface antigen subtypes adw and ayw, are currently under investigation in healthy and renal failure non responder patients: results to date are mixed [6, 56]. Pre-S components have been found to be more immunogenic than simple S containing vaccines by some authors, as they could overcome genetically defined unresponsiveness and protect even against emerging HBV mutants (G145R vaccine related mutations in 5% of vaccinated infants) resistant to anti-S antibodies [56].
Multiple attempts have been made to enhance the low immune response rate to the hepatitis B vaccine among patients with ESRD.
These attempts include recommendations as in table 4 [1, 5-6, 9-10, 14, 17, 20-21, 23, 26-27, 35, 37, 40-43, 47, 49-50, 54, 57-63].
|
Intramuscular:
Single dose (20μg) (3 doses)
Double dose (40μg) (3 doses) Adjuvants-immunostimulants (IL-2, IFN-α, G-M CSF, thymopentine, levamisole) Intradermal: 5-10 μg per 7-15 days (4-8 doses)
Intramuscular booster dose: 20-40μg in 6-12 months
additional if fall of antibody titer  10 mIU/ml 10 mIU/ml |
According to some authors, general measures associated with the elimination of "uremic" factors mentioned above, should be taken, in order to decrease the effect on immune defenses: erythropoietin and vitamin administration, hyperparathyroidism and iron overload treatment, use of more biocompatible dialysis material, efficient dialysis and better nutrition [17-19, 21, 58, 64]. Other investigators question the efficacy of such measures [27].
Incomplete protection and response variability are reported in chronic renal failure and haemodialysis patients, vaccinated against Hepatitis B by the classical intramuscular route [1, 22-23, 27, 40-41, 43, 47, 49, 57, 65-67].
In contrast to intramuscular administration, intradermal administration (the vaccine antigen remains trapped for long periods in the dermis, leading to longer macrophage dependent T cell stimulation and consequent higher immune response, with the aid of specific cells-relatively immature dendritic Langerhans cells) of small multiple doses, tried first in healthy and later in uremic non-responder patients, has proved to be simple, safe and relatively non-expensive method of HB vaccination, with, sometimes absolute, success in producing protective and lasting antibody titers in dialysis patients [1, 11, 21, 40, 43, 46, 48-49, 52, 57-58, 65, 68]. This method enables costs to be reduced enough to become manageable for dialysis patients [21, 40, 43, 46, 49, 52, 53, 58, 65, 68-70]. In our studies, protective levels were achieved even in patients not responsive to multiple-double quantity intramuscular doses. These findings have been confirmed by others [21, 52, 57, 65, 68, 70].
The degree of renal failure was not found to correlate to the attained level of immune response. Haemodialysis patients and renal failure patients not yet on dialysis reacted similarly to the injected antigen and produced similar levels of antibody "Fig. (1), (2)" [68].
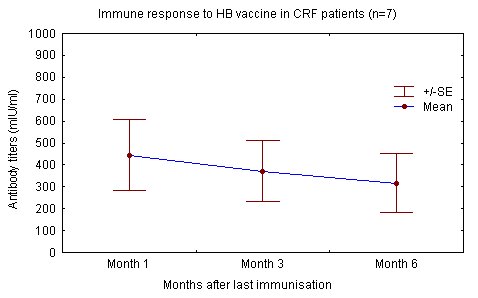
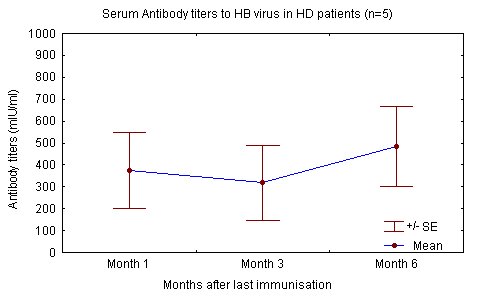
In contrast others have reported that as with IM administration, efficacy is lower in haemodialysis patients, even after vaccination with twice the number of doses used by our group. These differences may be explained by variations in application, population age, time in dialysis and dialysis adequacy [19, 21, 43, 48-49, 52]. Mettang et al and others also point out the importance of a strict intradermal vaccination technique (table 5) [21, 40, 49, 58].
| Intradermal (vs. Intramuscular) |
|
Less vaccine (economy)
Difficult technique More doses 100% response Earlier response Lower peak antibody levels Need for more frequent boosters? |
Our studies did not show factors associated with low or no response to the vaccine such as age, sex, body weight, degree of renal failure, time on haemodialysis, secondary hyperparathyroidism, HCV carrier state, dialysis modalities, serum albumin, hematocrit and hemoglobin levels, number of transfusions and erythropoietin treatment [5, 13, 18, 22-23, 27, 45, 47, 49, 54, 66]. Other authors insist on the importance of these factors as well as of the dialysis modality on the immune response (CAPD patients showing better response rates compared to haemodialysis patients) [1, 12, 19-23, 27, 50, 58, 71]. Our patients, vaccinated by the ID protocol, gradually lose adequate protection, after the first year, in contrast to those receiving IM vaccination (table 5).
Both routes were equally efficient in the first 12 months of observation "Fig. (3)" [11, 13, 21, 27, 40, 43, 52-53, 57-58]. Shorter follow up, longer initial vaccination times, alone or combined with higher total vaccine doses, besides the different approach to the booster method (IM or ID), could explain these differences [21, 40, 43, 52-53, 57-58].
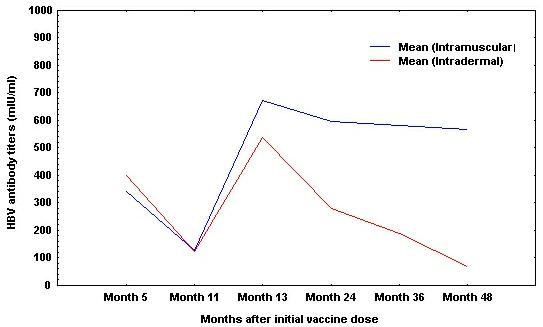
Peak antibody titers after ID vaccination are weaker than those achieved by intramuscular administration (table 5) [21, 40, 48, 53, 58, 65, 68, 70].
Although the ID method produces a universal and quick initial seroconversion and higher seroprotection rate in haemodialysis patients, compared to the IM route, seroprotection disappears quicker compared to the IM vaccination [40, 43, 53]. This is the reason why an increased need for boosters with ID method is noted (table 5) [57-58].
Despite seroprotection loss, patients can remain adequately protected, as long as frequent (yearly) titer measurements are taken, proving the long-term efficiency of the method. Consequently, timing for additional booster has to be closely followed-up when ID vaccination is applied [40, 53, 57].
Intramuscular vaccination against HBV together with ID administration of the vaccine to non responders to IM vaccination, using small total antigenic loads and limited numbers of inoculations, together with frequent, yearly, antibody level measurements and booster administration whenever protective levels were not detected, succeeded in preventing the appearance of new HB cases in our unit [21, 26, 68, 70]. Thus, vaccination against hepatitis B with recombinant (S) vaccine, first with the IM method and then, in non responders to IM, with the ID method, protects immunocompromised end stage renal disease patients as efficiently as it does in the healthy population [1, 12, 21, 26, 43, 57-58].
Renal failure patients with low producing IL-10 genotypes, which show no-response to hepatitis B vaccines may harbor an immune defect that is genetically defined. T cell CD4+ dysfunction and abnormal antigen presenting cells are the main causes of this immune defect.
This low or no response to HB vaccine can serve as an index of immune suppression in dialysis in patients and poor dialysis adequacy. Accordingly depressed immunity is also considered an additional, together with inadequate dialysis, inflammation and resulting malnutrition, risk factor for atherosclerosis, poor cardiovascular outcome and survival in ESRD.
Anemia correction by r-erythropoietin associated to lymphocyte subsets changes (increase in T helper/suppressor ratio) as well as better dialysis biocompatibility both contribute to better immune response to vaccination.
Both Intramuscular and Intradermal vaccination against hepatitis B have been used with variable efficiency in haemodialysis. The combination of IM and ID (for non responders) vaccination protocols succeeded, in some studies, in protecting up to 100% of the renal failure population.
Intradermal antigen presentation lasts longer, possible because it mobilizes dendritic Langerhans cells, leading to a more sustained stimulus that overcomes the "uremic" immune defect.
Immune response strength to Hepatitis B vaccine in haemodialysis patients is equivalent when immunization is conducted via either the IM or ID methods. However, later, antibody titers are found significantly lower in the ID immunization group. Consequently, these patients need more frequent, yearly, serum HBV(S)Ab measurements and booster doses when titers are found unprotective.
Multiple intradermal vaccination against HB virus, using a smaller total vaccine dose, is a safe, quick, cost/effective and successful approach that can be used in all susceptible predialysis and dialysis patients. The disadvantage to this method is that specific antibody titers decrease over time, thus necessitating the administration of additional boosters.
Second generation recombinant HB vaccines, intradermally administered, can overcome the immune defect in all renal failure patients. This ID vaccination protocol can successfully be used to vaccinate non-responder renal failure or ESRD patients until other methods have been proven effective. Third generation recombinant HB vaccines with S, pre-S1 and immunodominant domain pre-S2 genes have been shown to have promise and may eventually replace ID vaccination. Alternatively adjuvants for strong and specific stimulation of the immune response may provide enhanced vaccination efficacy.
Lecture based on the review: Recombinant Hepatitis B Vaccination in Renal Failure. Patients by D. Vlassopoulos in: Curr Pharm Biotechnology, 4(2): 141-151, 2003.
[2] Descamps-Latscha, B. and Chatenoud, L. T cells and B cells in chronic renal failure. (1996) Semin. Nephrol., 16(3), 183-191.
[3] Girndt, M.; Kohler, H.; Schiedhelm-Weick, E.; Schlaak, J. F.; Meyer Zum Buschenfelde, K. -H. and Fleischer, B. Production of interleukin-6, tumor necrosis factor α and interleukin-10 in vitro correlates with the clinical immune defect in chronic hemodialysis patients. (1995) Kidney Int., 47, 559-565.
[4] Girndt, M.; Sester, M.; Sester, U.; Kaul, H and Kohler, H. Defective expression of B7-2 (CD86) on monocytes of dialysis patients correlates to the uremia-associated immune defect. (2001) Kidney Int., 59, 1382-1389.
[5] Peces, R.; de la Torre, M.; Alcazar, R. and Urra, J.M. Prospective analysis of the factors influencing the antibody response to Hepatitis B vaccine in hemodialysis patients. (1997) Am. J. Kidney Dis., 29(2), 239-245.
[6] Haubitz, M.; Ehlerding, G.; Beigel, A.; Heuer, U.; Hemmerling, A.E. and Thoma, H.A. Clinical experience with a new recombinant hepatitis B vaccine in previous non-responders with chronic renal insufficiency. (1996) Clin. Nephrol., 45(3), 180-182.
[7] Girndt, M.; Sester, U.; Sester, M.; Deman, E.; Kaul, H and Kohler, H. The interleukin-10 promoter genotype determines clinical immune function in hemodialysis patients. (2001) Kidney Int., 60, 2385-2391.
[8] Van Riemsdijk-van Overbeeke, I.C.; Baan, C.C.; Knoop, C.J.; Loonen, E.H.M.; Zietse, R. and Weimar, W. Quantitative flow cytometry shows activation of TNF- α system but not of the IL-2 system at the single cell level in renal replacement therapy. (2001) Nephrol. Dial. Transplant., 16, 1430-1435.
[9] Stachowski, J.; Pollok, M.; Barth, C.; Maciejewski, J. and Baldamus, C. A. Non-responsiveness to hepatitis B vaccination in haemodialysis patients: association with impaired TCR/CD3 antigen receptor expression regulating co-stimulatory processes in antigen presentation and recognition. (1994) Nephrol. Dial. Transplant., 9, 144-152.
[10] Katkow, W.N. and Dienstag, J.L. Prevention and therapy of viral hepatitis. (1991) Semin. Liver Dis., 11(2), 165-174.
[11] Vlassopoulos, D.; Magana, P.; Hadjiyannakos, D.; Spyropoulou, M.; Lilis, D.; Stavropoulou, C. and Hadjiconstantinou, V. Factors involved in low response to HBV vaccine in health and end-stage renal failure. (1998) Nephrol. Dial. Transplant., 13, A 191.
[12] Pol, S.; Legendre, C.; Mattlinger, B.; Berthelot, P. and Kreis, H. Genetic basis to hepatitis B vaccine in hemodialyzed patients. (1990) J. Hepatol., 11, 385-387.
[13] Vlassopoulos, D.; Arvanitis, D.; Lilis D.; Louizou, K. and Hadjiconstantinou, V. Hepatitis B vaccination in ESRD. 8 year follow up. Factors involved in the immune response. (1999) Nephrol. Dial. Transplant., 14, A114.
[14] Caillat-Zucman, S.; Gimenez, J.-J.; Wambergue, F.; Albouze, G.; Lebkiri, B.; Naret, C.; Moynot, A.; Jungers, P. and Bach, J.-F. Distinct HLA class II alleles determine antibody response to vaccination with hepatitis B surface antigen. (1998) Kidney Int., 53, 1626-1630.
[15] Kruskall, M.S.; Alper, C.A.; Awdeh, Z.; Yunis, E.J. and Marcus-Bagley, D. The immune response to hepatitis B vaccine in humans: inheritance patterns in families. (1992) J. Exp. Med., 175, 495-502.
[16] Rahman, F.; Dahmen, A.; Herzog-Hauff, S.; Bocher, W.O.; Galle, P.R. and Lohr, H.F. Cellular and humoral responses induced by intradermal or intramuscular vaccination with the major Hepatitis B surface antigen. (2000) Hepatology, 31, 521-527.
[17] Vanholder, R.; Van Loo, A.; Dhondt, A.M.; De Smet, R. and Ringoir, S. Influence of uremia and haemodialysis on host defense and infection. (1996) Nephrol. Dial. Transplant., 11, 593-598.
[18] Gaciong, Z.; Alexiewicz, J.M. and, Massry, S.G. Impaired in vivo antibody production in CRF rats: Role of secondary hyperparathyroidism. (1991) Kidney Int., 40, 862-867.
[19] Fernandez, E.; Betriu, M.A.; Gomez, R. and Montoliu, J. Response to the hepatitis B virus vaccine in haemodialysis patients: influence of malnutrition and its importance as a risk factor for morbidity and mortality. (1996) Nephrol. Dial. Transplant., 11, 1559-1563.
[20] Fivush, B.A. and Neu, A.M. Immunization guidelines for pediatric renal diseases. (1998) Seminars in Nephrology, 18(3), 256-263.
[21] Fabrizi, F.; Andrulli, S.; Bacchini, G.; Corti, M. and Locatelli, F. Intradermal versus intramuscular hepatitis B vaccination in non-responding chronic dialysis patients: a prospective randomized study with cost-effectiveness evaluation. (1997) Nephrol. Dial. Transplant., 12,1204-1211.
[22] Navarro, J.F.; Teruel, J.L.; Mateos, M. and Ortuno, J. Hepatitis C virus infection decreases the effective antibody response to hepatitis B vaccine in haemodialysis patients. (1994) Clin. Nephrol., 41(22),113-116.
[23] Navarro, J.F.; Teruel, J.L.; Mateos, M.; Marcen, R. and Ortuno, J. Antibody level after hepatitis B vaccination in haemodialysis patients: influence of hepatitis C virus infection. (1996) Am. J. Nephrol., 16, 95-97.
[24] Guerin, A.; Buisson, Y.; Nutini, M.T.; Saliou, P.; London, G. and Marchais, S. Response to vaccination against tetanus in chronic haemodialyzed patients. (1992) Nephrol. Dial. Transplant., 7, 323-326.
[25] Girndt, M.; Pietsch, M. and Kohler, H. Tetanus immunization and its association to hepatitis B vaccination in patients with chronic renal failure. (1995) Am. J. Kidney Dis., 26(3), 454-460.
[26] Jungers, P. Vaccination contre l’hépatite B dans les centres d’hémodialyse. (1998) Nephrologie, 19, 37-38.
[27] Buti, M.; Viladomiu, L.; Jardi, R.; Olmos, A.; Rodriguez, J.A.; Bartolome, J.; Esteban, R. and Guardia, J. Long-term immunogenicity and efficacy of hepatitis B vaccine in haemodialysis patients. (1992) Am. J. Nephrol., 12, 144-147.
[28] Kreft, B.; Klouche, M.; Kreft, R.; Kirchner, H and Sack, K. Low efficiency of active immunization against diphtheria in chronic hemodialysis patients. (1997) Kidney Int., 52, 212-216.
[29] Shinefield, H.; Black, S.; Fattom, A. and Horwith, G. Use of a Staphylococcus aureus conjugate vaccine in patients receiving haemodialysis. (2002) N. Engl. J. Med., 346(7), 491-496.
[30] Sever, M.S.; Yildiz, A.; Eraksoy, H.; Badur S.; Yuksel-Onel D.; Gorcin B.; Turk S. and Erkoc R. Immune response to Haemophilus influenzae type B vaccination in renal transplant recipients with well-functioning allografts. (1999) Nephron, 81, 55-59.
[31] Stark, K.; Gunther, M.; Schonfeld, C. and Tullius S.G. Immunizations in solid-organ transplant recipients. (2002) Lancet, 359, 957-965.
[32] Kruger S.; Seyfarth M.; Sack K. and Kreft B. Defective immune response to tetanus toxoid in hemodialysis patients and its association with diphtheria vaccination. (1999) Vaccine, 17, 1145-1150.
[33] Lefebure, A.F.; Verpooten, G.A.; Couttenye, M.M. and DeBroe, M.E. Immunogenicity of a recombinant DNA hepatitis B vaccine in renal transplant patients. (1993) Vaccine, 11(4), 397-399.
[34] Miller, E.R.; Alter, M.J. and Tokars, J.I. Protective effect of hepatitis B vaccine in chronic haemodialysis patients. (1999) Am. J. Kidney Dis., 33(2), 356-360.
[35] Lewis-Ximenez, L.L.; Oliveira, J.M.; Mercadante, L.A.C.; De Castro, L.; Santa Catharina, W.; Stuver, S. and Yoshida, C.F.T. Serological and vaccination profile of haemodialysis patients during an outbreak of hepatitis B virus infection. (2001) Nephron, 87, 19-26.
[36] Jibani, M.M.; Heptonstall, J,; Walker, A.M.; Bloodworth, L.O. and Howard, A.J. Hepatitis B immunization in UK renal units: failure to put policy into practice. (1994) Nephrol. Dial. Transplant., 9, 1765-1768.
[37] Kohler, H. Hepatitis B immunization in dialysis patients-is it worthwhile? (1994) Nephrol. Dial. Transplant., 9, 1719-1720.
[38] Crosnier, J.; Jungers, P.; Courouce, A.M.; Laplanche, A.; Benhamou, E.; Degos, F.; Lacour, B.; Prunet, P.; Cerisier, Y. and Guesry, P. Randomized placebo- controlled trial of hepatitis B surface antigen vaccine in French haemodialysis units: II, haemodialysis patients. (1981) Lancet, 1(8224), 797-800.
[39] Stevens, C.E.; Alter, H.J.; Taylor, P.E.; Zhang E.A.; Harley E.J.; Szmuness W. and the Dialysis Vaccine Trial Study Group. Hepatitis B vaccine in patients receiving haemodialysis. Immunogenicity and efficacy. (1984) N. Engl. J. Med., 311, 496-501.
[40] Propst, T.; Propst, A.; Lhotta, K.; Vogel, W. and Konig, P. Reinforced intradermal Hepatitis B vaccination in haemodialysis patients is superior in antibody response to intramuscular or subcutaneous vaccination. (1998) Amer. J. Kidney Dis., 32(6), 1041-1045.
[41] Peces, R. and Laures, A.S. Persistence of immunologic memory in long-term hemodialysis patients and healthcare workers given hepatitis B vaccine: role of a booster dose on antibody response. (2001) Nephron, 89, 172-176.
[42] Drachman, R.; Isacsohn, M.; Rudensky, B. and Drukker, A. Vaccination against hepatitis B in children and adolescent patients on dialysis. (1989) Nephrol. Dial. Transplant., 4, 372-374.
[43] Chang, P.C.; Schrander-van der Meer, A.M.; van Dorp, W.T. and van Leer, E. Intracutaneous versus intramuscular hepatitis B vaccination in primary non-responding haemodialysis patients. (1996) Nephrol. Dial. Transplant., 11, 191-193.
[44] Szmuness, W.; Stevens, C.E.; Harley, E.J.; Zang, E.A.; Alter, H.J.; Taylor, P.E.; De Vera, A.; Chen, G.T.S.; Kellner, A. and Dialysis Vaccine Trial Study Group. (1982) N. Engl. J. Med., 307, 1481-1486.
[45] Dukes, C.S.; Street, A.C.; Starling, J.F. and Hamilton J.D. Hepatitis B vaccination and booster in predialysis patients: a 4-year analysis. (1993) Vaccine, 11(12), 1229-1232.
[46] Nagafuchi, S. and Kashiwagi, S. Reversal by intradermal hepatitis B vaccination of unresponsiveness to HBsAg. (1987) Lancet, ii, 1522-1523.
[47] Kohler, H.; Arnold, W.; Renschin, G.; Dormeyer, H. -H. and Meyer Zum Buschenfelde, K. -H. Active hepatitis B vaccination of dialysis patients and medical staff. (1984) Kidney Int., 25, 124-128.
[48] Zucherman, A.J. Appraisal of intradermal immunization against hepatitis B. (1987) Lancet, i, 435-436.
[49] Mettang, T.; Schenk, U.; Thomas, S.; Machleidt, C.; Kiefer, T.; Fischer, F. -P. and Kuhlmannn, U. (1996) Nephron, 72, 192-196.
[50] Mitwalli, A. Responsiveness to hepatitis B vaccine in immunocompromised patients by doubling the dose scheduling. (1996) Nephron, 73, 417-420.
[51] Carletti, P.; Bibiano, L.; Boggi, R.; Bordoni, E.; Ricciatti, A.M.; Della Bella, S.; Pauri, P.; Salvoni, G. and Mioli, V.A. HBV infection in haemodialysis patients: monitoring and prevention. (1992) Nephron, 61, 269-270.
[52] Poux, J.M.; Ranger, S.; Lagarde, C.; Benevent, D.; Denis, F. and Leroux-Robert, C. Efficacy of intradermal injection of recombinant hepatitis B vaccine in dialysis patients. (1994) Nephrol. Dial. Transplant., 9, 1213-1214.
[53] Ono, K. and Kashiwagi, S. Complete seroconversion by low-dose intradermal injection of recombinant hepatitis B Vaccine in haemodialysis patients. (1991) Nephron, 58, 47-51.
[54] Mauri, J.M.; Valles, M. and the collaborative group of Girona. Effects of recombinant interleukin-2 and revaccination for hepatitis B in previously vaccinated, non-responder, chronic uraemic patients. (1997) Nephrol. Dial. Transplant., 12, 729-732.
[55] Bruguera, M.; Cremades, M.; Rodicio, J.; Alcazar, J.M.; Oliver, A.; Del Rio, G. and Esteban-Mur, R. Immunogenicity of a yeast-derived hepatitis B vaccine in haemodialysis patients. (1989) Am J Med, 87(suppl. 3A), 30S-32S.
[56] Zuckerman, J.N.; Sabin, C.; Craig, F.M.; Williams, A. and Zuckerman, A.J. Immune response to a new hepatitis B vaccine in healthcare workers who had not responded to standard vaccine: randomized double blind dose-response study. (1997) B.M.J., 314, 329-333.
[57] Marangi, A.L.; Giordano, R.; Montanaro, A.; De Padova, F.; Schiavone, M.G.; Dongiovani, G. and Basile, C. Hepatitis B virus infection in chronic uremia: Long-term follow-up of a two- step integrated protocol of vaccination. (1994) Amer. J. Kidney Dis., 23, 537-542.
[58] Charest, A.F.; McDougall, J. and Goldstein, M.B. A randomized comparison of intradermal and intramuscular vaccination against hepatitis B virus in incident chronic haemodialysis patients. (2000) Am. J. Kidney Dis., 36(5), 976-982.
[59] Evans, T.G.; Schiff, M.; Graves, B.; Agosti J.; Barritt M. L.; Garner D. and Holley J.L. The safety and efficacy of GM-CSF as an adjuvant in hepatitis B vaccination of chronic haemodialysis patients who have failed primary vaccination. (2000) Clin. Nephrol., 54(2), 138-142.
[60] Brodersen, H.P.; Holtkamp, W.; Larbig, D.; Beckers, B.; Thiery, J.; Lautenschlager, J.; Probst, H. -J.; Ropertz, S. and Yavari, A. Zinc supplementation and hepatitis B vaccination in chronic haemodialysis patients: a multicenter study. (1995) Nephrol. Dial. Transplant., 10,1780-1783.
[61] Hibberd, P.L. and Rubin, R.H. Immunization strategies for the Immunocompromised Host: The Need for Immunoadjuvants. (1989) Ann. Intern. Med., 110(12), 955-956.
[62] Ayli, M.D.; Ensari, C.; Ayli, M.; Mandiroglu, F. and Mut, S. Effect of oral Levamisol supplementation to Hepatitis B vaccination on the rate of immune response in chronic hemodialysis patients. (2000) Nephron, 84, 291-292.
[63] Anandh, U.; Bastani, B. and Ballal, S. Granulocyte-Macrophage Colony-Stimulating Factor as an adjuvant to Hepatitis B vaccination in maintenance haemodialysis patients. (2000) Am. J. Nephrol., 20, 53-56.
[64] Sennesael, J.J.; Van Der Niepen, P. and Verbeelen, D. L. Treatment with recombinant human erythropoietin increases antibody titers after hepatitis B vaccination in dialysis patients. (1991) Kidney Int., 40, 121-128.
[65] Vlassopoulos, D.; Arvanitis, D.; Lilis, D.; Katopodis, K.; Louizou, K. and Hadjiconstantinou, V. Low repeated doses of intradermal hepatitis B vaccine produce protective levels of antibodies in haemodialysis patients, non responding to usual and double multiple doses. (1994) J. Nephrol., 7(6), 340-341.
[66] Arvanitis, D.; Vlassopoulos, D.; Lilis, D.; Dardioti, V.; Noussias, C.; Magana, P.; Louizou, K. and Hadjiconstantinou, V. Long term protection of haemodialysis patients from HBV, following response to conventional vaccination. (1996) Nephrol. Dial. Transplant., 11, A 182.
[67] Gesemann, M. and Scheiermann, N. Quantification of hepatitis B vaccine-induced antibodies as a predictor of anti-HBs persistence. (1995) Vaccine, 13(5), 443-447.
[68] Vlassopoulos, D.; Arvanitis, D.; Lilis D.; Hadjiyannakos, D.; Louizou, K. and Hadjiconstantinou, V. Complete success of intradermal vaccination against hepatitis B in advanced chronic renal failure and haemodialysis patients. (1997) Renal Failure, 19(3), 455-460.
[69] Miller, K.; Gibbs, R.; Mulligan, M.; Nutman, T. and Francis, D. Intradermal hepatitis B virus vaccine: immunogenicity and side effects in adults. (1983) Lancet, ii, 1454-1456.
[70] Vlassopoulos, D.; Arvanitis, D.; Lilis, D.; Louizou, K. and Hadjiconstantinou, V. Lower long-term efficiency of intradermal hepatitis B vaccine compared to the intramuscular route in haemodialysis patients. (1999) Int. J. Artif. Org., 22(11), 739-743.
[71] Birmingham, D.J.; Shen, X.P.; Hartman, J.A.; Dillon, J.J. and Hebert, L.A. Effect of chronic human recombinant erythropoietin therapy on antibody responses to immunization in chronic haemodialysis patients. (1996) Kidney Int., 50, 543-549.
[72] Vlassopoulos, D.; Arvanitis, D.; Lilis, D.; Noussias, C.; Magana, P.; Logothetis, E.; Katopodis, K. and Hadjiconstantinou, V. Intradermal vaccination against hepatitis B; full response of chronic renal failure and haemodialysis patients. (1995) Nephrol. Dial. Transplant., 10, 991.
[73] Coursaget, P.; Yvonnet, B.; Gilks, W.R.; Wang, C.C.; Day, N.E.; Chiron, J. –P. and Diop-Mar, I. Scheduling of revaccination against hepatitis B virus. (1991) Lancet, 337, 1180-1183.
| ABREVIATIONS
HB=Hepatitis B HBV= Hepatitis B virus HB (S) Ag=Hepatitis B surface antigen HD=Haemodialysis CRF=Chronic Renal Failure ESRD= end stage renal disease IM=Intramuscular ID=Intradermal CAPD=Continuous Ambulatory Peritoneal Dialysis IL=Interleukin IFN=Interferon TNF=Tumor Necrosis Factor G-M CSF= Granulocyte-Macrophage Colony Stimulating Factor Kt/V=dialysis adequacy index CDC=Center for Disease Control APC=Antigen Presenting Cell MHC= Major Histocompatibility Complex TCR=T Cell Receptor ICAM=Intracellular Adhesion Molecule HLA=Histocompatibility Locus A Th=T helper HCV= Hepatitis C Virus CTL=Cytotoxic T Lymphocytes |