
|
Paneles de Discussión
Paneais de Discussio |
Post-menopausal Osteoporosis in the Dialysis PatientJosé R. Weisinger and Ezequiel Bellorin-FontDivision of Nephrology, Hospital Universitario de Caracas, Universidad Central de Venezuela, Caracas, Venezuela.Key Words: Osteoporosis, bone mineral density, hemodialysis, hormone replacement therapy, bisphosphonates, SERM Abbreviations:
Abstract
Purpose of the review:
Osteoporosis is the most prevalent bone disorder in the general population, particularly in the middle and older age groups. Although more than half of the prevalent dialysis population is within these age groups, little concern has been given to the possible role of estrogen deficiency in the pathogenesis of bone disease in end stage renal disease (ESRD). The purpose of this review is to summarize the recent published evidence that supports a potential role of the postmenopausal state in the pathogenesis of bone disease in ESRD and their implications for treatment. Recent findings Recent studies have shown that although the risk factors for fracture in ESRD are similar to the general population, the incidence is three to four folds higher. The high prevalence of older population, the frequently observed premature amenorrhea and early menopause in dialysis patients may play a role. Similarly, the proportion of ESRD women receiving hormone replacement therapy (HRT) is at least three times lower than the general population. Recent evidence on the risk of HRT therapy should caution about its use in ESRD patients. New evidence suggest that selective estrogen receptor modulators (SERM) may increase bone mass without significant secondary effects. Other alternatives, such as the use of bisphosphonates, should be considered with caution due to the risk of excessive suppression of bone turnover, worsening or favoring the development of adynamic bone disease. Conclusions Osteoporosis should be recognized as an important entity that may modify the current conception of renal osteodystrophy in postmenopausal patients with ESRD. Further clinical studies are needed in order to propose strategies that may reduce the impact of postmenopausal osteoporosis in the dialysis population. Introduction Little concern has been given to the possible role of estrogen deficiency in the pathogenesis of uremic bone disease or the existence of post-menopausal osteoporosis in end-stage renal disease (ESRD) patients. The term renal osteodystrophy, widely accepted to define the bone lesions associated with chronic renal failure includes diseases that affect the control of bone remodeling, like high turnover bone disease caused basically by secondary hyperparathyroidism, or low turnover in the form of adynamic bone disease and osteomalacia, usually associated to vitamin D deficiency or aluminum toxicity 1. Although the prevalence of menopause in women on dialysis is very high and premature amenorrhea occurs frequently in young women with ESRD, post-menopausal osteoporosis has not been traditionally included under the term of renal osteodystrophy. This may be explained by the fact that the usual forms of renal osteodystrophy develop at a faster rate and represent a major clinical problem, dominating the contribution that postmenopausal osteoporosis could have in the bone alterations observed in the female uremic patient 2. But, it seems possible that post-menopausal osteoporosis increases the burden of bone alterations in patients already affected by the multiple factors that determine renal osteodystrophy. Influence of age and gender of the dialysis population: According to the 2002 United States Renal Data System (USRDS) report 3, 38.5 % of the prevalent dialysis population in USA is in the age range of 45 to 65 years. Similarly, in the European countries reporting to the ERA-EDTA Registry 4 and the Registry of the Latin American Society of Nephrology 5 the prevalence of patients in dialysis in those age groups ranges between 35 to 43 % and 37 to 44.8 %, respectively. Furthermore, 45 to 47 % of the patients in these age groups are female, suggesting that in this particular ESRD population there may be an increase in the risk factors for osteoporotic bone fractures as occurs in the general population. Risk of fractures among patients with end-stage renal disease and dialysis: Several studies have suggested that patients with ESRD are at increased risk for bone fractures. It has been shown, that like in the general population, the risk for fracture increases with age, female gender, Caucasian race, lower body mass index, and decreased bone mass. 6-7. However, ESRD patients have an increased risk for hip and vertebral fractures compared to the general population. Although it has been proposed that factors such as renal osteodystrophy and metabolic acidosis may determine the increased risk for fractures observed in these patients, it is possible that the relatively high incidence and prevalence of ESRD in the middle age and older population may also play an important role. Decreased bone mineral density (BMD) has been well described among patients with ESRD, but relatively little attention has been paid to the occurrence of fractures. A cross-sectional study in hemodialyzed Japanese men reported that almost 21 % of them had vertebral fractures 8. Using data from the USRDS, Alem et al described that the overall incidence of hip fracture among patients who had undergone dialysis between 1989 and 1996 was about fourfold higher than expected in the general population 9. Nevertheless, renal transplant patients exceed the dialysis risk of hip fracture during the first 1-3 years after transplantation 10. It is unclear what could be the role of customary bone densitometry measurement in the prevention or management of uremic patients who suffer osteoporosis related fractures 11. Although the majority favors that a low BMD can predict the incidence of hip or vertebral fractures 8,12, recent evidence shows that BMD analysis by dual x-ray absorptiometry (DEXA) or heel ultrasound, does not identify patients with dialysis-dependent renal failure who have fractures 13. Similarly, a recent large epidemiological study in patients with mild to moderate chronic renal insufficiency demonstrated that subjects with worse renal function had significantly lower femoral BMD, but this association was explained by confounding factors, principally gender, age and weight 14. Several authors have addressed the risk factors associated with the relatively high incidence of vertebral or hip fractures among hemodialysis patients. A recent study indicates that low radial BMD but not vertebral BMD, increased serum levels of alkaline phosphatase, and lower oral calcium carbonate intake, were important predictors of hip fractures among 183 Japanese patients on dialysis 15. Coco et al evaluated 1,272 patients on hemodialysis between 1988 and 1998 and found that those with low PTH values were more likely to sustain a hip fracture than patients with higher PTH levels 16. Similarly, Atsumi et al in Japan showed that hemodialysis patients with low PTH values had greater vertebral fracture risk than those with hyperparathyroidism 8. At the same time, a multivariate analysis of the USRDS has shown that age (per increasing decade), female gender, body mass index, race, and the presence of peripheral vascular disease were independently associated with increase rate of hip fracture 17. Gynecological issues in the uremic women: Early amenorrhea and hypoestrogenism in young dialysis patients Most of the gynecological interest in women with ESRD treated or not with dialysis has been related to the problem of reduced fertility and libido. In the early eighties it was recognized that uremic women have endocrine disturbances leading to menstrual and fertility disorders. It was postulated that a defect in the hypothalamic regulation of gonadothrophin secretion would result in lower estradiol peaks, lower follicle stimulating hormone (FSH)/luteinizing hormone ratios and higher prolactin concentration leading to anovulatory cycles and persistent amenorrhea. In addition, hyperprolactinemia was suggested as the cause of infertility and reduced sexual desire in these patients 18. Thus, women with chronic renal disease are frequently hypoestrogenic as a result of the disease or its treatment. These alterations in ovarian function and fertility that are commonly observed during dialysis improve after a successful renal transplant. Cochrane et al 19 questioned 100 women with chronic renal failure (26 of them on chronic dialysis) about different gynecological aspects. Menstrual disorders were present in 85 % of the patients. Menopause was found in one third of the patients, but only two of these women (6%) had been offered hormonal replacement therapy (HRT). Interestingly, this situation was not perceived to be a problem by the treating nephrologists. Another study in 66 women on dialysis younger than 55 years of age, showed that 58% were amenorrheic, and 28% postmenopausal. In the postmenopausal women group, only 5 % were receiving HRT 20. Stehman-Breen et al evaluated 1,499 women from the USRDS Dialysis Morbidity and Mortality Study, and found that the overall prevalence of HRT prescription was 10.8 % 21 compared to 34 and 33 % in the normal population in USA and Britain, respectively 22-23 Similarly, a recent study in Australia reported that menopause occurred earlier in women on dialysis than in the normal population and that an important group of postmenopausal women with vasomotor symptoms, vaginal dryness and mood changes were not offered HRT, as normal women do 24. A recent study in our center has also shown that only 11.3% of a group of 53 post-menopausal women in dialysis were receiving regular HRT 25. Therefore, as table 1 shows, it seems to be no doubt that the management of menopause in the dialysis population differs from that in normal women, particularly with regard to the prescription of HRT and perhaps, the recognition of menopause and other manifestations of gonadal failure as important aspects that require consideration. The reasons why these problems are somewhat overlooked are not clear. It is possible that besides the lack of attention of the treating physicians, women with ESRD are usually stoical and rarely complain about gynecological problems that may seem trivial in comparison to their renal disease and the burden of dialysis. As a consequence, HRT is not a frequently discussed issue between the patients and their physicians 26. 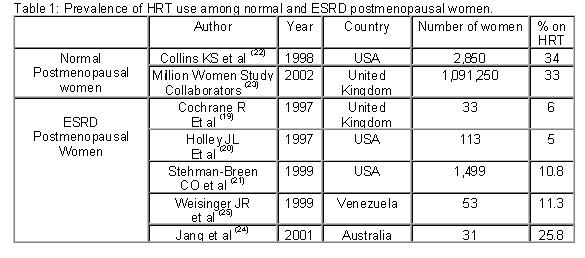 Previous studies have shown that young non-uremic women with anovulatory cycles have lower bone mass compared with regularly menstruating women 27-28. We have found similar results in young women (less than 50 years old) on dialysis 29. Indeed, in our study women with persistent amenorrhea showed significantly lower BMD when compared with a similar group of dialysis patients with regular menstruation. Interestingly, the patients with persistent amenorrhea demonstrated significantly lower serum estrogen levels, increased bone formation and resorption markers, independent of the serum levels of PTH, suggesting a higher bone turnover probably secondary to hypoestrogenemia. Estrogen metabolism in uremic women: Implications for HRT. Although the adverse effects of hypoestrogenism, and the beneficial effects of estrogens replacement on postmenopausal osteoporosis have been widely recognized, the mechanisms are not fully defined. Osteoblasts have estrogen receptors and respond directly or indirectly, inducing the production of growth factors such as IGF and TGF-β. Estrogen deficiency increases the production of IL-1, IL6, IL-11 and TNF- Estrogen may also affect bone metabolism through an effect on PTH synthesis. Indeed, parathyroid glands express estrogen receptors that respond to physiologically relevant doses of estradiol increasing PTH mRNA and PTH secretion 33 and modulating PTH action on bone. Indeed, in-vitro studies have demonstrated that estrogen inhibits PTH-stimulated osteoclast formation by directly affecting hemopoietic blasts cell precursors by a cAMP-mediated mechanism 34. We have recently shown that a large proportion of dialysis women below 50 years had persistent amenorrhea and estradiol levels significantly lower than age-matched women with normal menstruation 29. A large proportion of them had persistent amenorrhea with estradiol levels significantly lower than identical women with normal menstruations. Nevertheless, the absolute estradiol values were within the normal limits for women with normal renal function. Previous studies have demonstrated that total and free estradiol levels in patients with ESRD are higher at baseline and after estradiol administration when compared to the control population, suggesting that renal failure may decrease estradiol catabolism and affect the pharmacokinetics of exogenous estradiol 35. Although the role of uremia in the peripheral action of estradiol has not been completely defined, postmenopausal women may have estradiol levels within the accepted normal range, in the presence of inappropriate elevated FSH levels, that characterizes a postmenopausal status. These observations bring into account the issue of HRT in the uremic postmenopausal women. Clinical evidence in favor of a role of estrogen in uremic bone disease in women has been suggested by Matuszkiewicz-Rowinska et al 36 in a study of a small group of postmenopausal dialysis women. Treatment with transdermal estradiol and cyclic addition of norestisterone acetate significantly increased lumbar spine BMD after one year. Nevertheless, the issue of HRT with opposed estrogen-progestin therapy in uremic women is still controversial, especially after the recent Women’s Health Initiative (WHI) Study demonstrating increased risk of breast cancer, pulmonary embolism, coronary and cerebrovascular disease after a long-term combination of estrogen and progestin in normal postmenopausal women 37. In this randomized controlled primary prevention trial the estrogen plus progesterone treatment reduced the observed hip and vertebral fracture rate and colon cancer by one third. Unfortunately, the rate of women experiencing coronary hearth disease increased by 29 %, venous thromboembolism was 2-fold greater, stroke rates were increased by 41 %, and invasive breast cancer increased by 26 %. Thus, we have to wait for conclusive studies and perhaps to consider the use of other treatment alternatives before HRT can be prescribed freely to ESRD women, a subset of patients with potentially increased risk associated to this type of hormone therapy. Selective estrogen receptor modulators (SERM) in chronic renal failure. Several alternatives are now available for women who cannot receive, or refuse to take, estrogen. Two potential estrogen-like therapies include raloxifene and tamoxifen. These are SERMs with tissue-selective estrogen agonist and antagonist properties 38. In the large Multiple Outcomes of Raloxifene Evaluation (MORE) trial of 7,705 women with osteoporosis, 3 years of treatment with raloxifene at the dose of 60 mg/day increased BMD at the lumbar spine by 2.7% and decreased the incidence of vertebral fractures by 41%, without affecting the occurrence of hip or other nonvertebral fractures 39. Similarly, raloxifene has been shown to decrease serum lipid values in postmenopausal women 40. This effect in the lipoprotein profile could be relevant in patients on dialysis, since cardiovascular disease is the single best predictor of mortality in patients with end-stage renal failure, as it accounts for almost 50% of deaths. 41 We performed a double-blind controlled study on the effect of raloxifene on bone and lipid metabolism in ESRD postmenopausal women with severe osteopenia in hemodialysis 42. After one year of treatment with raloxifene there was an increase in BMD at the lumbar spine, together with a decrease in markers of bone resorption (serum pyridinoline), whereas no changes were observed in the placebo group. In addition, there was also a significant decrease in LDL-cholesterol levels with no changes in total cholesterol or triglycerides. No side effects, including venous thrombosis, pulmonary thromboembolism or clotting problems with the vascular accesses or dialysis catheters were observed after one year on raloxifene. A further analysis demonstrated that the estrogen receptor (ER) polymorphism could predict the response of BMD to raloxifene 43. In summary, raloxifene has proven to be an effective drug in terms of bone mineral and lipid metabolism in dialysis patients, with no significant side effects. However, the long-term effects of this drug remain to be analyzed. Tibolone Tibolone represents another possible option in ESRD and dialysis women. This drug, a different tissue-specific compound that has a favorable effect on bone, vaginal tissue, climateric symptoms, mood and sexual well-being in post-menopausal women, without estrogen-like stimulation of the endometrium and breast. Although to our knowledge no studies with tibolone have been reported drug in ESRD patients, a study in postmenopausal women with different degrees of renal function demonstrated that the pharmacokinetics parameters of tibolone and its primary metabolites were similar in subjects with normal to severely impaired renal function 44 Herbal medicine and menopause Despite the great achievements in medicine and development of new drugs, millions of people are turning back to traditional herbal medicines in order to prevent or treat a host of illnesses 45. Between 1990 and 1997 the use of complementary and alternative treatments increased 380%, and 61.5 % of users of these products were not disclosing their use to their treating physicians. One of these compounds, phytoestrogens binds to both estrogen receptors α and β and exert estrogen-like effects. These are naturally occurring plant compounds that are present in soybeans as isoflavones and in flaxseed as lignans. Studies in human subjects with different types of chronic renal disease, soy protein and flaxseed appear to moderate proteinuria and preserve renal function 46-47. Nevertheless, the long-term safety and efficacy of dietary soy and flaxseed need to be evaluated before it can be recommended as an alternative to other traditional modes of therapy. A recent review on the evidence and safety of products like black cohosh, ginseng, chastetree, dong quai, evening primrose oil, soy products and natural hormones caution about the quality of these products and conclude that none of the herbal remedies advertised for the treatment of menopausal symptoms can be recommended without hesitation in ESRD patients until the scientific evidence has improved 48 Bisphosphonates in end stage renal failure Although bisphosphonates are widely used to reduce fracture risk in patients with osteoporosis, the use of bisphosphonates to treat dialysis patients with osteoporosis have never been tested prospectively. Renal excretion is the major route of elimination of these drugs, but different studies have shown that intravenous clodronate or ibandronate are removed efficiently from the circulation by dialysis and that the total clearance in hemodialysis patients on a dialysis day is not very different from that in healthy subjects. 40-50 These compounds could reduce the high bone turnover of severe secondary hyperparathyroidism, as has been shown in recent studies in which bone deposition of clodronate was related to bone turnover, However, even in the patients with normal PTH, significant bone uptake of the drug still occurs 51. Therefore, the indiscriminate use of the compounds may significantly suppress bone turnover, favoring the development or worsening of adynamic bone disease. Role of estrogen in the bone loss of male patients on dialysis Different cross-sectional and longitudinal studies have shown that age-associated declines in non-uremic male BMD are more directly related to declining estrogens levels than to declining androgen levels, and that estrogen levels correlated better than testosterone with BMD 52. At the same time, vertebral fractures occur in the same proportion in men and women on hemodialysis. For these reasons, it will be necessary to design studies to evaluate the role of sex steroids in the bone loss of male dialysis patients. Conclusions The successful use of renal replacement therapy has resulted in longer survival and inclusion of older patients with ESRD, including people with other significant preexisting illnesses. Bone disease and fractures represent an important problem in this group of patients, but its relationship with ESRD remains complex. Postmenopausal osteoporosis has been recognized as an important entity associated to renal osteodystrophy and efforts have been started to tackle the reduced BMD and increased fracture rate. Although fracture risk reduction data are needed, preliminary observations on the effect of HRT as well as estrogen receptor modulators on BMD, bone resorption markers and serum lipids in uremic women are encouraging. Nevertheless, due to the pharmacokinetics of these drugs in ESRD and the recent observations of increased side effects of some of these compounds, clinical studies to guide the prescription of these drugs in women on dialysis, including short and long-term studies are needed. For now, we should be discussing gynecological issues with our women patients, and pending the studies, design treatment strategies, including well-tested and newer drug regimens in the management of osteoporosis and other postmenopausal symptoms in our dialysis patients. Acknowledgments This study was supported by grant G-97-008808 of the Fondo Nacional de Ciencia, Tecnología y Innovación de Venezuela (FONACIT) and Fundarenal-HUC. References
|