Diapositiva 15 de 48
Notas:
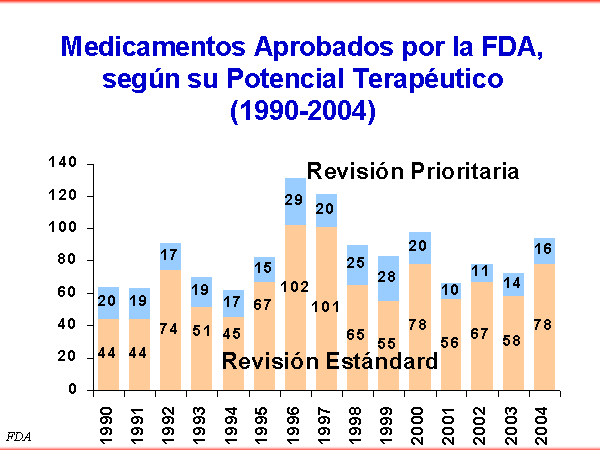
En términos globales, solamente el 22% de los nuevos medicamentos aprobados por la FDA americana desde 1990 a 2004 fueron medicamentos que parecía, en el momento de su aprobación, una mejora significativa sobre los productos ya disponibles en el mercado.
NOTE: An NDA can be granted to a drug that is a new molecular entity, new ester, new salt, or other noncovalent derivative, new formulation, new combination, new manufacturer, new indication (Beginning in 1994, new indication were tracked as efficacy supplements, not as NDAs), and drug already marketed, but without an approved NDA.
The FDA has a Classification of NDAs (new drug approval) by therapeutic potential
Priority Review: Significant improvement compared to marketed products, in the treatment, diagnosis, or prevention of a disease
Standard Review: The drug appears to have therapeutic qualities similar to those of one or more already marketed drugs.